Page 229 - Managing Global Warming
P. 229
188 Managing Global Warming
4.5.3 Ceramic fuels
Ceramic fuels have high melting points, good dimensional and radiation stability and
are chemically compatible with most coolants and cladding (sheath) materials. In
addition to melting point, the thermal conductivity of a fuel is a critical property that
affects the operating temperature of the fuel (the highest temperature in a reactor is the
fuel centerline temperature, for hollow pellets it will be the internal wall temperature).
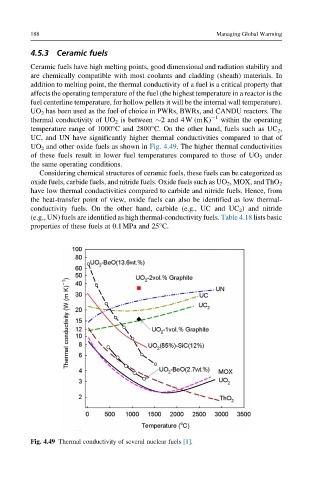
UO 2 has been used as the fuel of choice in PWRs, BWRs, and CANDU reactors. The
thermal conductivity of UO 2 is between 2 and 4W (mK) 1 within the operating
temperature range of 1000°C and 2800°C. On the other hand, fuels such as UC 2 ,
UC, and UN have significantly higher thermal conductivities compared to that of
UO 2 and other oxide fuels as shown in Fig. 4.49. The higher thermal conductivities
of these fuels result in lower fuel temperatures compared to those of UO 2 under
the same operating conditions.
Considering chemical structures of ceramic fuels, these fuels can be categorized as
oxide fuels, carbide fuels, and nitride fuels. Oxide fuels such as UO 2 , MOX, and ThO 2
have low thermal conductivities compared to carbide and nitride fuels. Hence, from
the heat-transfer point of view, oxide fuels can also be identified as low thermal-
conductivity fuels. On the other hand, carbide (e.g., UC and UC 2 ) and nitride
(e.g., UN) fuels are identified as high thermal-conductivity fuels. Table 4.18 lists basic
properties of these fuels at 0.1MPa and 25°C.
Fig. 4.49 Thermal conductivity of several nuclear fuels [1].