Page 200 - Manufacturing Engineering and Technology - Kalpakjian, Serope : Schmid, Steven R.
P. 200
Section 7.2 The Structure of Polymers I 79
(mostly amorphous) polymers. The degree of crystallinity also is affected by branch-
ing. A linear polymer can become highly crystalline, but a highly branched polymer
cannot, although it may develop some low level of crystallinity. It will never achieve
a high crystallite content, because the branches interfere with the alignment of the
chains into a regular crystal array.
Effects of Crystallinity. The mechanical and physical properties of polymers are
greatly influenced by the degree of crystallinity: as crystallinity increases, polymers
become stiffer, harder, less ductile, more dense, less rubbery, and more resistant to
solvents and heat (Fig. 7.6). The increase in density with increasing crystallinity is
called crystallization shrinkage and is caused by a more efficient packing of the mol-
ecules in the crystal lattice. For example, the highly crystalline form of polyethylene,
known as high-density polyethylene (HDPE), has a specific gravity in the range of
0.941 to 0.970 (80 to 95% crystalline). It is stronger, stiffer, tougher, and less ductile
than low-density polyethylene (LDPE), which is about 60 to 70% crystalline and
has a specific gravity of about 0.910 to 0.925.
Optical properties of polymers also are affected by the degree of crystallinity.
The reflection of light from the boundaries between the crystalline and the amor-
phous regions in the polymer causes opaqueness. Furthermore, because the index of
refraction is proportional to density, the greater the density difference between the
amorphous and crystalline phases, the greater is the opaqueness of the polymer.
Polymers that are completely amorphous can be transparent, such as polycarbonate
and acrylics.
7.2.3 Glass-transition Temperature
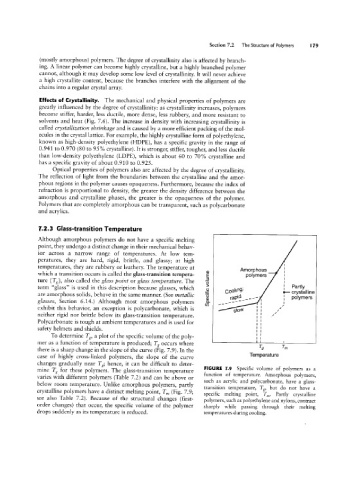
Although amorphous polymers do not have a specific melting
point, they undergo a distinct change in their mechanical behav-
ior across a narrow range of temperatures. At low tem-
peratures, they are hard, rigid, brittle, and glassy; at high
temperatures, they are rubbery or leathery. The temperature at
(D Amorphous
which a transition occurs is called the glass-transition tempera- 5 polymers
ture (Tg), also called the glass point or glass temperature. The §
term “glass” is used in this description because glasses, which Goomg-_ ;_ 5f';?;||ine
exhibit this behavior, an exception is polycarbonate, which is w posiymers
are amorphous solids, behave in the same manner. (See metallic
,aw-_\ ____
E;
glasses, Section 6.14.) Although most amorphous polymers cg- _,_--"'
neither rigid nor brittle below its glass-transition temperature. /
Polycarbonate is tough at ambient temperatures and is used for
safety helmets and shields.
To determine Tg, a plot of the specific volume of the poly-
mer as a function of temperature is produced; Tg occurs where '-lg Q-m
there is a sharp change in the slope of the curve (Fig. 7.9). In the
mperature
case of highly cross-linked polymers, the slope of the curve
changes gradually near Tg; hence, it can be difficult to deter-
mine Tg for these polymers. The glass-transition temperature FIGURE 7.9 Specific volume of polymers as a
function of temperature. Amorphous polymers,
varies with different polymers (Table 7.2) and can be above or
such as acrylic and polycarbonate, have a glass-
below room temperature. Unlike amorphous polymers, partly
transition temperature, Tg, but do not have a
crystalline polymers have a distinct melting point, Tm (Fig. 7.9;
specific melting point, Tm. Partly crystalline
see also Table 7.2). Because of the structural changes (first- polymers, such as polyethylene and nylons, contract
order changes) that occur, the specific volume of the polymer sharply while passing through their melting
drops suddenly as its temperature is reduced. temperatures during cooling.