Page 197 - Manufacturing Engineering and Technology - Kalpakjian, Serope : Schmid, Steven R.
P. 197
Chapter 7 Polymers: Structure, General Properties, and Applications
(a) Linear (b)Branched
e ar *
i ‘****. a
if *sg * *T * ii fi; it
in
Q
si
'S' 5' ‘ *I* il s*iz»§a*“ ir
,i*"**i»;;s»‘***‘ *Hifi* *i f *a*,1»* i l»
if f g i GV ‘ie
ar"§i**1~a;**§ii**Di 1* i*§§*¢ it *itiiiilllii
* z *U f in .B
*i¢s:'**"'~r;‘** Q ¢ #T
a
(c)Cross-linked (d) Network
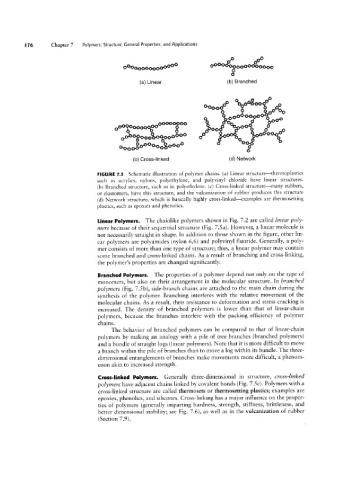
FIGURE 1.5 Schematic illustration of polymer chains. (a) Linear structure-thermoplastics
such as acrylics, nylons, polyethylene, and polyvinyl chloride have linear structures.
(b) Branched structure, such as in polyethylene. (c) Cross-linked structure-many rubbers,
or elastomers, have this structure, and the vulcanization of rubber produces this structure
(d) Network structure, which is basically highly cross-linked-examples are thermosetting
plastics, such as epoxies and phenolics.
Linear Polymers. The chainlike polymers shown in Fig. 7.2 are called linear poly-
mers because of their sequential structure (Fig. 7.5a). However, a linear molecule is
not necessarily straight in shape. In addition to those shown in the figure, other lin-
ear polymers are polyamides (nylon 6,6) and polyvinyl fluoride. Generally, a poly-
mer consists of more than one type of structure; thus, a linear polymer may contain
some branched and cross-linked chains. As a result of branching and cross-linking,
the polymer’s properties are changed significantly.
Branched Polymers. The properties of a polymer depend not only on the type of
monomers, but also on their arrangement in the molecular structure. In branched
polymers (Fig. 7.5b), side-branch chains are attached to the main chain during the
synthesis of the polymer. Branching interferes with the relative movement of the
molecular chains. As a result, their resistance to deformation and stress cracking is
increased. The density of branched polymers is lower than that of linear-chain
polymers, because the branches interfere with the packing efficiency of polymer
chains.
The behavior of branched polymers can be compared to that of linear-chain
polymers by making an analogy with a pile of tree branches (branched polymers)
and a bundle of straight logs (linear polymers). Note that it is more difficult to move
a branch within the pile of branches than to move a log within its bundle. The three-
dimensional entanglements of branches make movements more difficult, a phenom-
enon akin to increased strength.
Cross-linked Polymers. Generally three-dimensional in structure, cross-linked
polymers have adjacent chains linked by covalent bonds (Fig. 7.5c). Polymers with a
cross-linked structure are called thermosets or thermosetting plastics; examples are
epoxies, phenolics, and silicones. Cross-linking has a major influence on the proper-
ties of polymers (generally imparting hardness, strength, stiffness, brittleness, and
better dimensional stability; see Fig. 7.6), as well as in the vulcanization of rubber
(Section 7.9).