Page 161 - Materials Science and Engineering An Introduction
P. 161
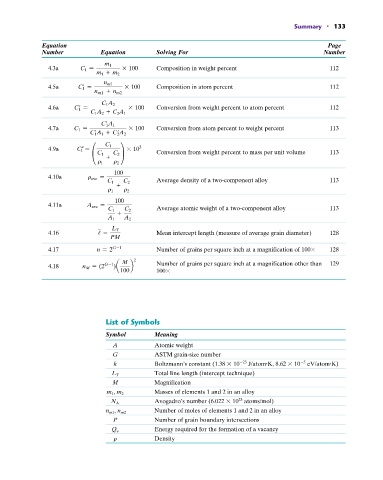
Summary • 133
Equation Page
Number Equation Solving For Number
m 1
4.3a C 1 = * 100 Composition in weight percent 112
m 1 + m 2
n m1
4.5a C 1 = * 100 Composition in atom percent 112
n m1 + n m2
C 1 A 2
4.6a C 1 = * 100 Conversion from weight percent to atom percent 112
C 1 A 2 + C 2 A 1
C 1 A 1
4.7a C 1 = * 100 Conversion from atom percent to weight percent 113
C 1 A 1 + C 2 A 2
C 1
3
4.9a C 1 = * 10
° C 1 C 2 ¢ Conversion from weight percent to mass per unit volume 113
+
r 1 r 2
100
4.10a r ave =
C 1 C 2 Average density of a two-component alloy 113
+
r 1 r 2
100
4.11a A ave =
C 1 C 2 Average atomic weight of a two-component alloy 113
+
A 1 A 2
4.16 / = L T Mean intercept length (measure of average grain diameter) 128
PM
4.17 n = 2 G-1 Number of grains per square inch at a magnification of 100 128
M 2 Number of grains per square inch at a magnification other than 129
4.18 n M = (2 G-1 )a b
100 100
List of Symbols
Symbol Meaning
A Atomic weight
G ASTM grain-size number
#
#
k Boltzmann’s constant (1.38 10 23 J/atom K, 8.62 10 eV/atom K)
5
L T Total line length (intercept technique)
M Magnification
m 1 , m 2 Masses of elements 1 and 2 in an alloy
23
N A Avogadro’s number (6.022 10 atoms/mol)
n m1 , n m2 Number of moles of elements 1 and 2 in an alloy
P Number of grain boundary intersections
Q y Energy required for the formation of a vacancy
r Density