Page 37 - Materials Science and Engineering An Introduction
P. 37
1.4 Classification of Materials • 9
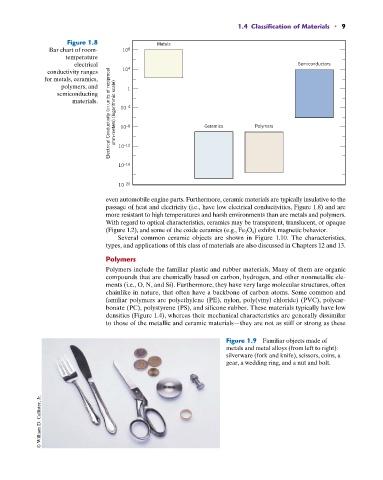
Figure 1.8 Metals
Bar chart of room- 10 8
temperature
electrical Semiconductors
Electrical Conductivity (in units of reciprocal ohm-meters) (logarithmic scale) 10 –4 Ceramics Polymers
conductivity ranges 10 4
for metals, ceramics,
polymers, and 1
semiconducting
materials.
–8
10
10
–16
10 –12
10 –20
even automobile engine parts. Furthermore, ceramic materials are typically insulative to the
passage of heat and electricity (i.e., have low electrical conductivities, Figure 1.8) and are
more resistant to high temperatures and harsh environments than are metals and polymers.
With regard to optical characteristics, ceramics may be transparent, translucent, or opaque
(Figure 1.2), and some of the oxide ceramics (e.g., Fe 3 O 4 ) exhibit magnetic behavior.
Several common ceramic objects are shown in Figure 1.10. The characteristics,
types, and applications of this class of materials are also discussed in Chapters 12 and 13.
Polymers
Polymers include the familiar plastic and rubber materials. Many of them are organic
compounds that are chemically based on carbon, hydrogen, and other nonmetallic ele-
ments (i.e., O, N, and Si). Furthermore, they have very large molecular structures, often
chainlike in nature, that often have a backbone of carbon atoms. Some common and
familiar polymers are polyethylene (PE), nylon, poly(vinyl chloride) (PVC), polycar-
bonate (PC), polystyrene (PS), and silicone rubber. These materials typically have low
densities (Figure 1.4), whereas their mechanical characteristics are generally dissimilar
to those of the metallic and ceramic materials—they are not as stiff or strong as these
Figure 1.9 Familiar objects made of
metals and metal alloys (from left to right):
silverware (fork and knife), scissors, coins, a
gear, a wedding ring, and a nut and bolt.
© William D. Callister, Jr.