Page 166 - Materials Chemistry, Second Edition
P. 166
153
References
2. Show the relationship between 4 1 and 4 3 crystallographic point group operations.
3. A metal alloy consists of 5 at.% Au and 95 at.% Pt; calculate the composition in terms of wt%. How
many atoms of gold will be present per cubic meter of the alloy?
4. What are the differences between amorphous and crystalline materials? Cite examples of each.
5. Prove that the theoretical packing densities for BCC, FCC, and HCP are 68%, 74%, and 74%,
respectively.
6. Describe some techniques used to fabricate amorphous or crystalline materials.
7. What is the difference between point groups and space groups?
8. Is it possible to have a material that is both an ionic solid and a molecular solid? Explain your
reasoning, and cite examples of such hybrids (if possible).
9. Consulting a Periodic Table and Table of Atomic Radii, what atoms would be suitable (a) interstitial
dopants and (b) substitutional dopants within a Mn lattice? Show your calculations and rationale.
10. Using diagrams, determine whether (111) or (110) planes are more densely packed with atoms for
FCC crystals.
11. Explain why the dislocation density of a single crystal is six times greater within 200 mm from the
surface, relative to its bulk structure. Would a surface oxide layer induce more dislocations to form in
a metal crystal, or insulate against dislocation formation? Explain.
12. Based on lattice parameters, explain why tetragonal, trigonal, and hexagonal crystals are often
dichroic, whereas orthorhombic, monoclinic, and triclinic crystals may exhibit trichroism.
13. What are the benefits of fuel cells, as compared to batteries and other “standard” power sources such
as fossil fuel power plants?
14. Why do acid-catalyzed and base-catalyzed sol-gel processes result in linear and branched metal
oxides, respectively?
15. Describe the unit cell symmetry/centering and crystallographic symmetry operations (incl. transla-
tional operations such as glide planes and screw axes) for each of the following space groups: (a) P6 3 /
m, (b) Fd 3, (c) Imma, (d) P6mm, (e) Fddd, (f) P2 1 /m.
16. For each of the crystallographic point groups represented in Q #15 above, determine whether the
crystal is piezoelectric (change in voltage (DV) in response to a change in pressure) and/or pyroelec-
tric (DV in response to a change in temperature). What are the governing symmetry elements that
determine whether a crystal will exhibit these properties?
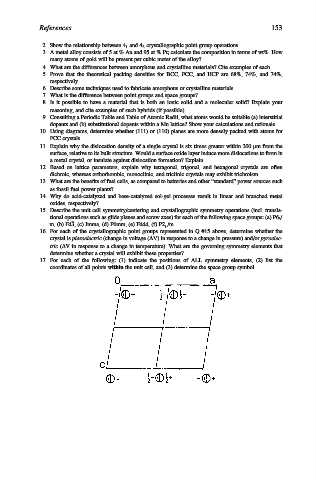
17. For each of the following: (1) indicate the positions of ALL symmetry elements, (2) list the
coordinates of all points within the unit cell, and (3) determine the space group symbol.