Page 168 - Materials Chemistry, Second Edition
P. 168
155
References
34. g-Al 2 O 3 , commonly used as a heterogeneous catalyst support, crystallizes in the Fd-3m space group.
Describe the multiplicity and fractional coordinates of O and Al ions in the crystal lattice.
35. Predict and explain whether nonzero intensities for the (111) and (110) reflections will be observed
from X-ray diffraction characterization of the following single crystals:
(a) Barium sodium niobate: Ba (4þx) Na (2 2x) Nb 10 O 30 (J. Chem. Phys. 1969, 50, 4352)
(b) Hexathorium fluoride hydrate: Th 6 F 24 H 2 O(Acta Cryst. B 1979, 35, 1763)
(c) Antimony gold yttrium: Au 3 Sb 4 Y 3 (Acta Cryst. B 1977, 33, 1579)
(d) Ruthenium (III) chloride: RuCl 3 (J. Chem. Soc. A 1967, 1038)
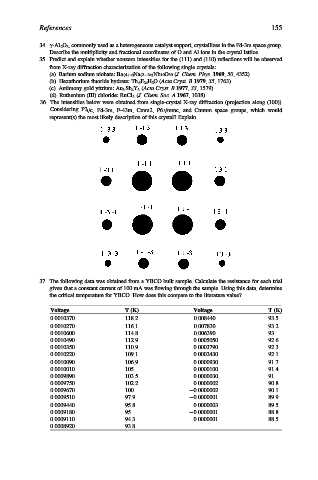
36. The intensities below were obtained from single-crystal X-ray diffraction (projection along (100)).
Considering P2 1 /c, Fd-3m, F-43m, Cmm2, P6 3 /mmc, and Cmmm space groups, which would
represent(s) the most likely description of this crystal? Explain.
37. The following data was obtained from a YBCO bulk sample. Calculate the resistance for each trial
given that a constant current of 100 mA was flowing through the sample. Using this data, determine
the critical temperature for YBCO. How does this compare to the literature value?
Voltage T (K) Voltage T (K)
0.0010370 118.2 0.008440 93.5
0.0010270 116.1 0.007830 93.2
0.0010600 114.8 0.006390 93
0.0010490 112.9 0.0005050 92.6
0.0010350 110.9 0.0003790 92.3
0.0010220 109.1 0.0002430 92.1
0.0010090 106.9 0.0000930 91.7
0.0010010 105 0.0000100 91.4
0.0009890 103.5 0.0000030 91
0.0009750 102.2 0.0000002 90.8
0.0009670 100 0.0000002 90.1
0.0009510 97.9 0.0000001 89.9
0.0009440 95.8 0.0000003 89.5
0.0009180 95 0.0000001 88.8
0.0009110 94.3 0.0000001 88.5
0.0008920 93.8