Page 173 - Materials Chemistry, Second Edition
P. 173
160 3 Metals
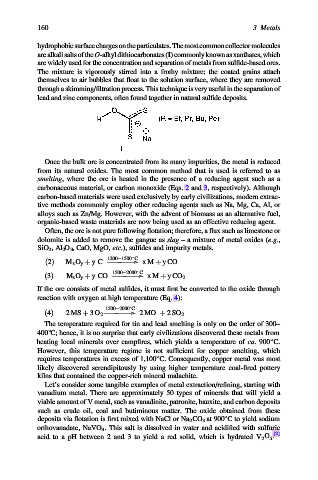
hydrophobicsurfacechargesontheparticulates.Themostcommoncollectormolecules
are alkalisaltsofthe O-alkyldithiocarbonates(I) commonlyknownasxanthates,which
are widely used for the concentration and separation of metals from sulfide-based ores.
The mixture is vigorously stirred into a frothy mixture; the coated grains attach
themselves to air bubbles that float to the solution surface, where they are removed
through a skimming/filtration process. This technique is very useful in the separation of
lead and zinc components, often found together in natural sulfide deposits.
Once the bulk ore is concentrated from its many impurities, the metal is reduced
from its natural oxides. The most common method that is used is referred to as
smelting, where the ore is heated in the presence of a reducing agent such as a
carbonaceous material, or carbon monoxide (Eqs. 2 and 3, respectively). Although
carbon-based materials were used exclusively by early civilizations, modern extrac-
tive methods commonly employ other reducing agents such as Na, Mg, Ca, Al, or
alloys such as Zn/Mg. However, with the advent of biomass as an alternative fuel,
organic-based waste materials are now being used as an effective reducing agent.
Often, the ore is not pure following flotation; therefore, a flux such as limestone or
dolomite is added to remove the gangue as slag – a mixture of metal oxides (e.g.,
SiO 2 ,Al 2 O 3 , CaO, MgO, etc.), sulfides and impurity metals.
1200 1500 C
ð2Þ M x O y þ yC ! xM þ yCO
1500 2000 C
ð3Þ M x O y þ yCO ! xM þ yCO 2
If the ore consists of metal sulfides, it must first be converted to the oxide through
reaction with oxygen at high temperature (Eq. 4):
1500 2000 C
ð4Þ 2MS þ 3O 2 ! 2MO þ 2SO 2
The temperature required for tin and lead smelting is only on the order of 300–
400 C; hence, it is no surprise that early civilizations discovered these metals from
heating local minerals over campfires, which yields a temperature of ca. 900 C.
However, this temperature regime is not sufficient for copper smelting, which
requires temperatures in excess of 1,100 C. Consequently, copper metal was most
likely discovered serendipitously by using higher temperature coal-fired pottery
kilns that contained the copper-rich mineral malachite.
Let’s consider some tangible examples of metal extraction/refining, starting with
vanadium metal. There are approximately 50 types of minerals that will yield a
viable amount of V metal, such as vanadinite, patronite, bauxite, and carbon deposits
such as crude oil, coal and butiminous matter. The oxide obtained from these
deposits via flotation is first mixed with NaCl or Na 2 CO 3 at 900 C to yield sodium
orthovanadate, NaVO 3 . This salt is dissolved in water and acidified with sulfuric
acid to a pH between 2 and 3 to yield a red solid, which is hydrated V 2 O 5 . [2]