Page 176 - Materials Chemistry, Second Edition
P. 176
163
3.1. Mining and Processing of Metals
(iii) Metal Recovery – the metal is recovered using electrolytic reduction of the
concentrated solution, which is known as electrowinning. This is suitable for
most metals, and is most commonly used for the alkali metals, rare earths, Pb,
Cu, Zn, Ni, Co, Au, Ag, Al, Cr, and Mn.
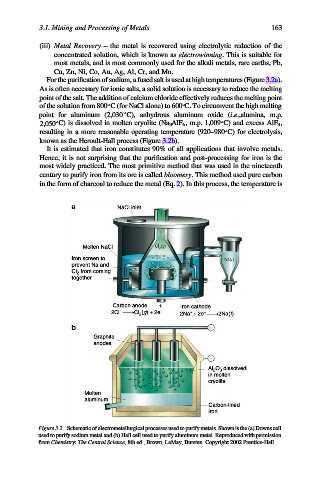
For the purification of sodium, a fused salt is used at high temperatures (Figure 3.2a).
As is often necessary for ionic salts, a solid solution is necessary to reduce the melting
point of the salt. The addition of calcium chloride effectively reduces the melting point
of the solution from 800 C (for NaCl alone) to 600 C. To circumvent the high melting
point for aluminum (2,030 C), anhydrous aluminum oxide (i.e.,alumina, m.p.
2,050 C) is dissolved in molten cryolite (Na 3 AlF 6 ,m.p. 1,009 C) and excess AlF 3 ,
resulting in a more reasonable operating temperature (920–980 C) for electrolysis,
known as the Heroult-Hall process (Figure 3.2b).
It is estimated that iron constitutes 90% of all applications that involve metals.
Hence, it is not surprising that the purification and post-processing for iron is the
most widely practiced. The most primitive method that was used in the nineteenth
century to purify iron from its ore is called bloomery. This method used pure carbon
in the form of charcoal to reduce the metal (Eq. 2). In this process, the temperature is
Figure 3.2. Schematic of electrometallurgical processes used to purify metals. Shown is the (a) Downs cell
used to purify sodium metal and (b) Hall cell used to purify aluminum metal. Reproduced with permission
from Chemistry: The Central Science, 8th ed., Brown, LeMay, Bursten. Copyright 2002 Prentice-Hall.