Page 67 - Materials Chemistry, Second Edition
P. 67
54 2 Solid-State Chemistry
BiO
SrO
CUO 2
Ca
CuO 2
SrO
c = 30.7 Å BiO
BiO
CuO
CUO 2 SrO
LaO BaO CuO 2
LaO CUO 2 Ca
c = 13.18 Å CUO 2 c = 11.6802 Å CUO 2 CuO 2
y
LaO
SrO
BaO
LaO
CUO 2 CUO BiO
b = 3.78Å b = 3.89Å b = 5.4Å
a = 3.78Å a = 3.82Å a = 5.4Å
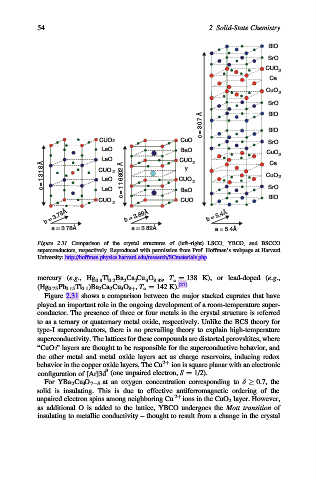
Figure 2.31. Comparison of the crystal structures of (left–right) LSCO, YBCO, and BSCCO
superconductors, respectively. Reproduced with permission from Prof. Hoffman’s webpage at Harvard
University: http://hoffman.physics.harvard.edu/research/SCmaterials.php.
mercury (e.g.,Hg 0.8 Tl 0.2 Ba 2 Ca 2 Cu 3 O 8.33 , T c ¼ 138 K), or lead-doped (e.g.,
(Hg 0.75 Pb 0.15 Tl 0.1 )Ba 2 Ca 2 Cu 3 O 8þ , T c ¼ 142 K). [27]
Figure 2.31 shows a comparison between the major stacked cuprates that have
played an important role in the ongoing development of a room-temperature super-
conductor. The presence of three or four metals in the crystal structure is referred
to as a ternary or quaternary metal oxide, respectively. Unlike the BCS theory for
type-I superconductors, there is no prevailing theory to explain high-temperature
superconductivity. The lattices for these compounds are distorted perovskites, where
“CuO 2 ” layers are thought to be responsible for the superconductive behavior, and
the other metal and metal oxide layers act as charge reservoirs, inducing redox
behavior in the copper oxide layers. The Cu 2þ ion is square planar with an electronic
9
configuration of [Ar]3d (one unpaired electron, S ¼ 1/2).
For YBa 2 Cu 3 O 7 d at an oxygen concentration corresponding to d 0.7, the
solid is insulating. This is due to effective antiferromagnetic ordering of the
unpaired electron spins among neighboring Cu 2þ ions in the CuO 2 layer. However,
as additional O is added to the lattice, YBCO undergoes the Mott transition of
insulating to metallic conductivity – thought to result from a change in the crystal