Page 62 - Materials Chemistry, Second Edition
P. 62
49
2.3. The Crystalline State
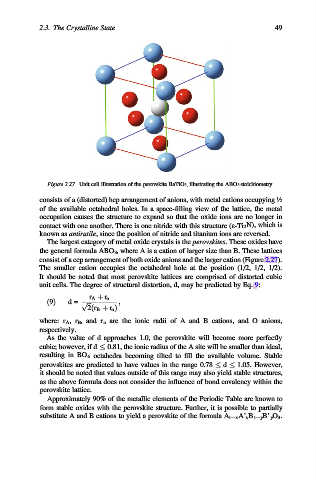
Figure 2.27. Unit cell illustration of the perovskite BaTiO 3 , illustrating the ABO 3 stoichiometry.
consists of a (distorted) hcp arrangement of anions, with metal cations occupying ½
of the available octahedral holes. In a space-filling view of the lattice, the metal
occupation causes the structure to expand so that the oxide ions are no longer in
contact with one another. There is one nitride with this structure (e-Ti 2 N), which is
known as antirutile, since the position of nitride and titanium ions are reversed.
The largest category of metal oxide crystals is the perovskites. These oxides have
the general formula ABO 3 , where A is a cation of larger size than B. These lattices
consist of a ccp arrangement of both oxide anions and the larger cation (Figure 2.27).
The smaller cation occupies the octahedral hole at the position (1/2, 1/2, 1/2).
It should be noted that most perovskite lattices are comprised of distorted cubic
unit cells. The degree of structural distortion, d, may be predicted by Eq. 9:
r A þ r o
ð9Þ d= p ffiffiffi ;
2ðr B þ r o Þ
where: r A ,r B , and r o are the ionic radii of A and B cations, and O anions,
respectively.
As the value of d approaches 1.0, the perovskite will become more perfectly
cubic; however, if d 0.81, the ionic radius of the A site will be smaller than ideal,
resulting in BO 6 octahedra becoming tilted to fill the available volume. Stable
perovskites are predicted to have values in the range 0.78 d 1.05. However,
it should be noted that values outside of this range may also yield stable structures,
as the above formula does not consider the influence of bond covalency within the
perovskite lattice.
Approximately 90% of the metallic elements of the Periodic Table are known to
form stable oxides with the perovskite structure. Further, it is possible to partially
substitute A and B cations to yield a perovskite of the formula A 1 x A’ x B 1 y B’ y O 3 .