Page 58 - Materials Chemistry, Second Edition
P. 58
45
2.3. The Crystalline State
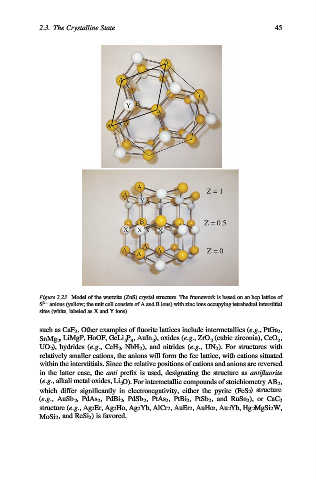
Figure 2.23. Model of the wurtzite (ZnS) crystal structure. The framework is based on an hcp lattice of
S 2 anions (yellow; the unit cell consists of A and B ions) with zinc ions occupying tetrahedral interstitial
sites (white, labeled as X and Y ions).
such as CaF 2 . Other examples of fluorite lattices include intermetallics (e.g., PtGa 2 ,
SnMg 2 , LiMgP, HoOF, GeLi 5 P 3 , AuIn 2 ), oxides (e.g., ZrO 2 (cubic zirconia), CeO 2 ,
UO 2 ), hydrides (e.g., CeH 2 , NbH 2 ), and nitrides (e.g.,UN 2 ). For structures with
relatively smaller cations, the anions will form the fcc lattice, with cations situated
within the interstitials. Since the relative positions of cations and anions are reversed
in the latter case, the anti prefix is used, designating the structure as antifluorite
(e.g., alkali metal oxides, Li 2 O). For intermetallic compounds of stoichiometry AB 2 ,
which differ significantly in electronegativity, either the pyrite (FeS 2 ) structure
(e.g., AuSb 2 , PdAs 2 ,PdBi 2 , PdSb 2 , PtAs 2 , PtBi 2 , PtSb 2 , and RuSn 2 ), or CaC 2
structure (e.g., Ag 2 Er, Ag 2 Ho, Ag 2 Yb, AlCr 2 , AuEr 2 , AuHo 2 ,Au 2 Yb, Hg 2 MgSi 2 W,
MoSi 2 , and ReSi 2 ) is favored.