Page 55 - Materials Chemistry, Second Edition
P. 55
42 2 Solid-State Chemistry
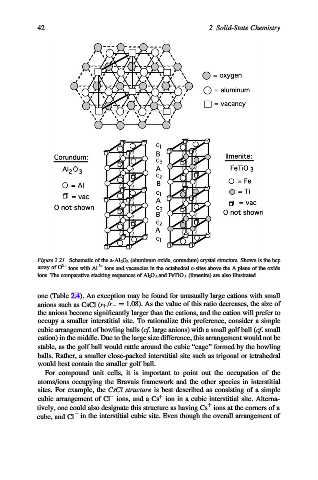
Figure 2.21. Schematic of the a-Al 2 O 3 (aluminum oxide, corundum) crystal structure. Shown is the hcp
array of O 2 ions with Al 3+ ions and vacancies in the octahedral c-sites above the A plane of the oxide
ions. The comparative stacking sequences of Al 2 O 3 and FeTiO 3 (ilmenite) are also illustrated.
one (Table 2.4). An exception may be found for unusually large cations with small
anions such as CsCl (r þ /r ¼ 1.08). As the value of this ratio decreases, the size of
the anions become significantly larger than the cations, and the cation will prefer to
occupy a smaller interstitial site. To rationalize this preference, consider a simple
cubic arrangement of bowling balls (cf. large anions) with a small golf ball (cf. small
cation) in the middle. Due to the large size difference, this arrangement would not be
stable, as the golf ball would rattle around the cubic “cage” formed by the bowling
balls. Rather, a smaller close-packed interstitial site such as trigonal or tetrahedral
would best contain the smaller golf ball.
For compound unit cells, it is important to point out the occupation of the
atoms/ions occupying the Bravais framework and the other species in interstitial
sites. For example, the CsCl structure is best described as consisting of a simple
þ
cubic arrangement of Cl ions, and a Cs ion in a cubic interstitial site. Alterna-
tively, one could also designate this structure as having Cs ions at the corners of a
þ
cube, and Cl in the interstitial cubic site. Even though the overall arrangement of