Page 54 - Materials Chemistry, Second Edition
P. 54
41
2.3. The Crystalline State
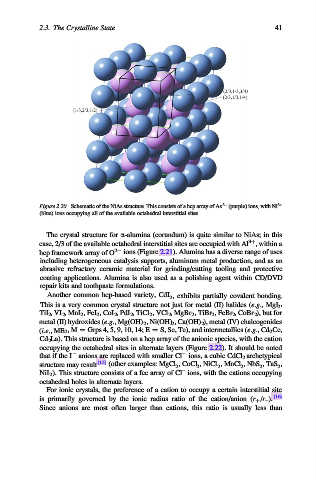
Figure 2.20. Schematic of the NiAs structure. This consists of a hcp array of As 3 (purple) ions, with Ni 3+
(blue) ions occupying all of the available octahedral interstitial sites.
The crystal structure for a-alumina (corundum) is quite similar to NiAs; in this
3þ
case, 2/3 of the available octahedral interstitial sites are occupied with Al , within a
hcp framework array of O 2 ions (Figure 2.21). Alumina has a diverse range of uses
including heterogeneous catalysis supports, aluminum metal production, and as an
abrasive refractory ceramic material for grinding/cutting tooling and protective
coating applications. Alumina is also used as a polishing agent within CD/DVD
repair kits and toothpaste formulations.
Another common hcp-based variety, CdI 2 , exhibits partially covalent bonding.
This is a very common crystal structure not just for metal (II) halides (e.g., MgI 2 ,
TiI 2 ,VI 2 , MnI 2 , FeI 2 , CoI 2 , PdI 2 , TiCl 2 , VCl 2 , MgBr 2 , TiBr 2 , FeBr 2 , CoBr 2 ), but for
metal (II) hydroxides (e.g., Mg(OH) 2 , Ni(OH) 2 , Ca(OH) 2 ), metal (IV) chalcogenides
(i.e., ME 2 ,M ¼ Grps 4, 5, 9, 10, 14; E ¼ S, Se, Te), and intermetallics (e.g., Cd 2 Ce,
Cd 2 La). This structure is based on a hcp array of the anionic species, with the cation
occupying the octahedral sites in alternate layers (Figure 2.22). It should be noted
that if the I anions are replaced with smaller Cl ions, a cubic CdCl 2 archetypical
structure may result [15] (other examples: MgCl 2 , CoCl 2 , NiCl 2 , MnCl 2 , NbS 2 , TaS 2 ,
NiI 2 ). This structure consists of a fcc array of Cl ions, with the cations occupying
octahedral holes in alternate layers.
For ionic crystals, the preference of a cation to occupy a certain interstitial site
is primarily governed by the ionic radius ratio of the cation/anion (r þ /r ). [16]
Since anions are most often larger than cations, this ratio is usually less than