Page 59 - Materials Chemistry, Second Edition
P. 59
46 2 Solid-State Chemistry
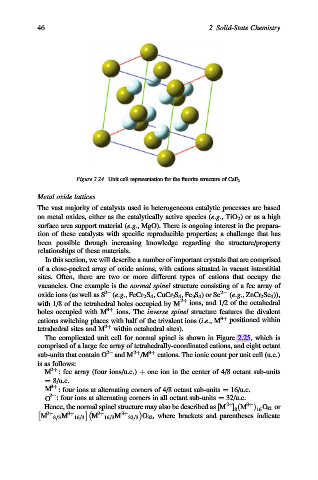
Figure 2.24. Unit cell representation for the fluorite structure of CaF 2.
Metal oxide lattices
The vast majority of catalysts used in heterogeneous catalytic processes are based
on metal oxides, either as the catalytically active species (e.g., TiO 2 ) or as a high
surface area support material (e.g., MgO). There is ongoing interest in the prepara-
tion of these catalysts with specific reproducible properties; a challenge that has
been possible through increasing knowledge regarding the structure/property
relationships of these materials.
In this section, we will describe a number of important crystals that are comprised
of a close-packed array of oxide anions, with cations situated in vacant interstitial
sites. Often, there are two or more different types of cations that occupy the
vacancies. One example is the normal spinel structure consisting of a fcc array of
oxide ions (as well as S 2 (e.g., FeCr 2 S 4 , CuCr 2 S 4 ,Fe 3 S 4 )or Se 2 (e.g., ZnCr 2 Se 4 )),
with 1/8 of the tetrahedral holes occupied by M 2þ ions, and 1/2 of the octahedral
holes occupied with M 3þ ions. The inverse spinel structure features the divalent
cations switching places with half of the trivalent ions (i.e.,M 3þ positioned within
tetrahedral sites and M 2þ within octahedral sites).
The complicated unit cell for normal spinel is shown in Figure 2.25, which is
comprised of a large fcc array of tetrahedrally-coordinated cations, and eight octant
2þ
sub-units that contain O 2 and M /M 3þ cations. The ionic count per unit cell (u.c.)
is as follows:
2þ
M : fcc array (four ions/u.c.) þ one ion in the center of 4/8 octant sub-units
¼ 8/u.c.
3þ
M : four ions at alternating corners of 4/8 octant sub-units ¼ 16/u.c.
2
O : four ions at alternating corners in all octant sub-units ¼ 32/u.c.
3þ
2þ
Hence, the normal spinel structure may also be described as ½M ðM Þ O 32 or
8 16
M 2þ 8=3 M 3þ 16=3 M 2þ 16=3 M 3þ 32=3 O 32 , where brackets and parentheses indicate