Page 56 - Materials Chemistry, Second Edition
P. 56
43
2.3. The Crystalline State
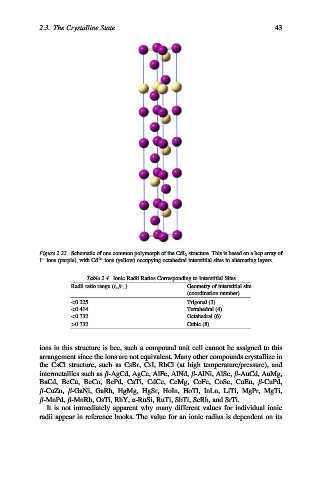
Figure 2.22. Schematic of one common polymorph of the CdI 2 structure. This is based on a hcp array of
I ions (purple), with Cd 2+ ions (yellow) occupying octahedral interstitial sites in alternating layers.
Table 2.4. Ionic Radii Ratios Corresponding to Interstitial Sites
Radii ratio range (r + /r ) Geometry of interstitial site
(coordination number)
<0.225 Trigonal (3)
<0.414 Tetrahedral (4)
<0.732 Octahedral (6)
>0.732 Cubic (8)
ions in this structure is bcc, such a compound unit cell cannot be assigned to this
arrangement since the ions are not equivalent. Many other compounds crystallize in
the CsCl structure, such as CsBr, CsI, RbCl (at high temperature/pressure), and
intermetallics such as b-AgCd, AgCe, AlFe, AlNd, b-AlNi, AlSc, b-AuCd, AuMg,
BaCd, BeCu, BeCo, BePd, CaTi, CdCe, CeMg, CoFe, CoSc, CuEu, b-CuPd,
b-CuZn, b-GaNi, GaRh, HgMg, HgSr, HoIn, HoTl, InLn, LiTi, MgPr, MgTi,
b-MnPd, b-MnRh, OsTi, RhY, a-RuSi, RuTi, SbTi, ScRh, and SrTi.
It is not immediately apparent why many different values for individual ionic
radii appear in reference books. The value for an ionic radius is dependent on its