Page 76 - Materials Chemistry, Second Edition
P. 76
63
2.3. The Crystalline State
possible for a triclinic crystal are P1 and P1. For monoclinic crystals, there
are three possible crystallographic point groups (2, m, and 2/m), which are combined
with varying combinations of lattice centering and glide planes/screw axes to
yield 13 possible space groups: P2, P2 1 , C2, Pm, Pc, Cm, Cc, P2/m, P2 1 /m, C2/m
(or B2/m), P2/c, P2 1 /c, and C2/c (or B2/b). More explicitly, the P2 space group would
represent a primitive monoclinic unit cell, with a twofold rotation axis parallel to
b ([010] direction). In comparison, the Cm space group indicates a C-centered unit cell
with a mirror plane perpendicular to b. There are 59 orthorhombic, 68 tetragonal,
25 trigonal, 27 hexagonal, and 36 cubic space groups for a total of 230.
In order to extract the relevant symmetry elements from a space group symbol,
the following procedure should be followed. First, the centering is readily deter-
mined from the first term of the symbol. The relevant crystal system may be
determined by comparing the remaining terms with the 32 crystallographic point
groups (Table 2.5), treating screw axes and glide planes as rotation axes and mirror
planes, respectively. For instance, the Pmmm space group implies that it is a
primitive unit cell; the mmm is found in Table 2.5 for an orthorhombic unit cell.
Hence, this would translate to a primitive orthorhombic unit cell. Next, using
Table 2.6 for an orthorhombic system, the Pmmm space group would be defined by
mirror planes perpendicular to each of the a, b,and c axes. As another example,
let’s consider a very common space group, P2 1 /c. This can be simplified to the 2/m
crystallographic point group, which is in the monoclinic crystal system. Hence,
this space group consists of a primitive monoclinic unit cell, with a twofold screw
axis parallel to b (rotation of 180 about b, followed by translation along b of 1/2
the unit cell distance). In addition, a c-glide plane is perpendicular to the screw axis
(ab mirror plane, with 1/2 translation along c). For a crystal classified within the F432
space group (e.g., sodium phosphate, Na 3 PO 4 ), we can determine this is comprised of a
fcc unit cell, with a fourfold rotation axis parallel to a, b,and c, a threefold rotation
axis parallel to the cube diagonal [111], and a twofold rotation axis parallel to the
ab plane [110].
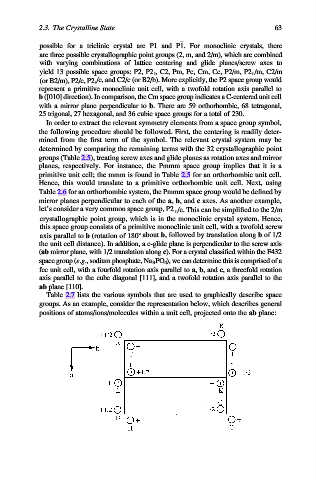
Table 2.7 lists the various symbols that are used to graphically describe space
groups. As an example, consider the representation below, which describes general
positions of atoms/ions/molecules within a unit cell, projected onto the ab plane: