Page 100 - Mechanical Behavior of Materials
P. 100
Section 3.6 Ceramics and Glasses 99
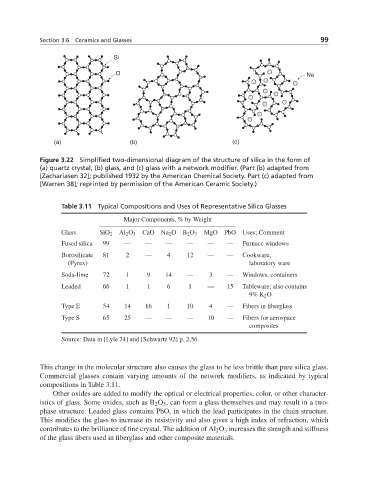
Si
O Na
(a) (b) (c)
Figure 3.22 Simplified two-dimensional diagram of the structure of silica in the form of
(a) quartz crystal, (b) glass, and (c) glass with a network modifier. (Part (b) adapted from
[Zachariasen 32]; published 1932 by the American Chemical Society. Part (c) adapted from
[Warren 38]; reprinted by permission of the American Ceramic Society.)
Table 3.11 Typical Compositions and Uses of Representative Silica Glasses
Major Components, % by Weight
Glass SiO 2 Al 2 O 3 CaO Na 2 O B 2 O 3 MgO PbO Uses; Comment
Fused silica 99 — — — — — — Furnace windows
Borosilicate 81 2 — 4 12 — — Cookware,
(Pyrex) laboratory ware
Soda-lime 72 1 9 14 — 3 — Windows, containers
Leaded 66 1 1 6 1 — 15 Tableware; also contains
9% K 2 O
Type E 54 14 16 1 10 4 — Fibers in fiberglass
Type S 65 25 — — — 10 — Fibers for aerospace
composites
Source: Data in [Lyle 74] and [Schwartz 92] p. 2.56.
This change in the molecular structure also causes the glass to be less brittle than pure silica glass.
Commercial glasses contain varying amounts of the network modifiers, as indicated by typical
compositions in Table 3.11.
Other oxides are added to modify the optical or electrical properties, color, or other character-
istics of glass. Some oxides, such as B 2 O 3 , can form a glass themselves and may result in a two-
phase structure. Leaded glass contains PbO, in which the lead participates in the chain structure.
This modifies the glass to increase its resistivity and also gives a high index of refraction, which
contributes to the brilliance of fine crystal. The addition of Al 2 O 3 increases the strength and stiffness
of the glass fibers used in fiberglass and other composite materials.