Page 97 - Mechanical Behavior of Materials
P. 97
96 Chapter 3 A Survey of Engineering Materials
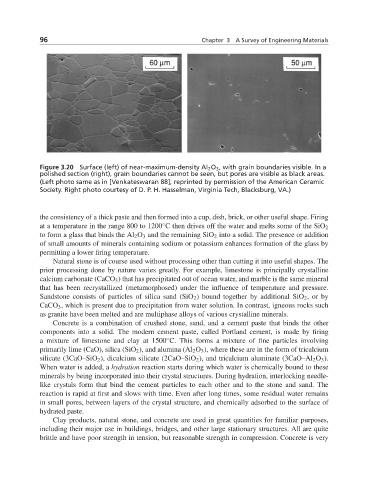
Figure 3.20 Surface (left) of near-maximum-density Al 2 O 3 , with grain boundaries visible. In a
polished section (right), grain boundaries cannot be seen, but pores are visible as black areas.
(Left photo same as in [Venkateswaran 88]; reprinted by permission of the American Ceramic
Society. Right photo courtesy of D. P. H. Hasselman, Virginia Tech, Blacksburg, VA.)
the consistency of a thick paste and then formed into a cup, dish, brick, or other useful shape. Firing
◦
at a temperature in the range 800 to 1200 C then drives off the water and melts some of the SiO 2
to form a glass that binds the Al 2 O 3 and the remaining SiO 2 into a solid. The presence or addition
of small amounts of minerals containing sodium or potassium enhances formation of the glass by
permitting a lower firing temperature.
Natural stone is of course used without processing other than cutting it into useful shapes. The
prior processing done by nature varies greatly. For example, limestone is principally crystalline
calcium carbonate (CaCO 3 ) that has precipitated out of ocean water, and marble is the same mineral
that has been recrystallized (metamorphosed) under the influence of temperature and pressure.
Sandstone consists of particles of silica sand (SiO 2 ) bound together by additional SiO 2 ,orby
CaCO 3 , which is present due to precipitation from water solution. In contrast, igneous rocks such
as granite have been melted and are multiphase alloys of various crystalline minerals.
Concrete is a combination of crushed stone, sand, and a cement paste that binds the other
components into a solid. The modern cement paste, called Portland cement, is made by firing
◦
a mixture of limestone and clay at 1500 C. This forms a mixture of fine particles involving
primarily lime (CaO), silica (SiO 2 ), and alumina (Al 2 O 3 ), where these are in the form of tricalcium
silicate (3CaO–SiO 2 ), dicalcium silicate (2CaO–SiO 2 ), and tricalcium aluminate (3CaO–Al 2 O 3 ).
When water is added, a hydration reaction starts during which water is chemically bound to these
minerals by being incorporated into their crystal structures. During hydration, interlocking needle-
like crystals form that bind the cement particles to each other and to the stone and sand. The
reaction is rapid at first and slows with time. Even after long times, some residual water remains
in small pores, between layers of the crystal structure, and chemically adsorbed to the surface of
hydrated paste.
Clay products, natural stone, and concrete are used in great quantities for familiar purposes,
including their major use in buildings, bridges, and other large stationary structures. All are quite
brittle and have poor strength in tension, but reasonable strength in compression. Concrete is very