Page 92 - Mechanical Behavior of Materials
P. 92
Section 3.5 Polymers 91
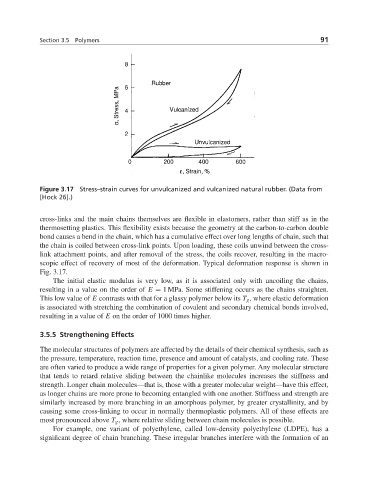
Figure 3.17 Stress–strain curves for unvulcanized and vulcanized natural rubber. (Data from
[Hock 26].)
cross-links and the main chains themselves are flexible in elastomers, rather than stiff as in the
thermosetting plastics. This flexibility exists because the geometry at the carbon-to-carbon double
bond causes a bend in the chain, which has a cumulative effect over long lengths of chain, such that
the chain is coiled between cross-link points. Upon loading, these coils unwind between the cross-
link attachment points, and after removal of the stress, the coils recover, resulting in the macro-
scopic effect of recovery of most of the deformation. Typical deformation response is shown in
Fig. 3.17.
The initial elastic modulus is very low, as it is associated only with uncoiling the chains,
resulting in a value on the order of E = 1 MPa. Some stiffening occurs as the chains straighten.
This low value of E contrasts with that for a glassy polymer below its T g , where elastic deformation
is associated with stretching the combination of covalent and secondary chemical bonds involved,
resulting in a value of E on the order of 1000 times higher.
3.5.5 Strengthening Effects
The molecular structures of polymers are affected by the details of their chemical synthesis, such as
the pressure, temperature, reaction time, presence and amount of catalysts, and cooling rate. These
are often varied to produce a wide range of properties for a given polymer. Any molecular structure
that tends to retard relative sliding between the chainlike molecules increases the stiffness and
strength. Longer chain molecules—that is, those with a greater molecular weight—have this effect,
as longer chains are more prone to becoming entangled with one another. Stiffness and strength are
similarly increased by more branching in an amorphous polymer, by greater crystallinity, and by
causing some cross-linking to occur in normally thermoplastic polymers. All of these effects are
most pronounced above T g , where relative sliding between chain molecules is possible.
For example, one variant of polyethylene, called low-density polyethylene (LDPE), has a
significant degree of chain branching. These irregular branches interfere with the formation of an