Page 44 - Mechanical Behavior of Materials
P. 44
Section 2.2 Bonding in Solids 43
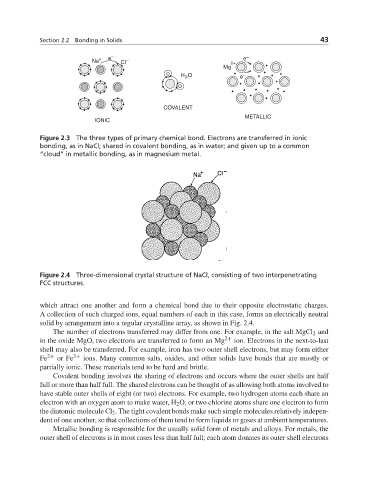
Na + e – Cl – 2+ e –
Mg
H O e –
2
COVALENT
METALLIC
IONIC
Figure 2.3 The three types of primary chemical bond. Electrons are transferred in ionic
bonding, as in NaCl; shared in covalent bonding, as in water; and given up to a common
“cloud” in metallic bonding, as in magnesium metal.
Figure 2.4 Three-dimensional crystal structure of NaCl, consisting of two interpenetrating
FCC structures.
which attract one another and form a chemical bond due to their opposite electrostatic charges.
A collection of such charged ions, equal numbers of each in this case, forms an electrically neutral
solid by arrangement into a regular crystalline array, as shown in Fig. 2.4.
The number of electrons transferred may differ from one. For example, in the salt MgCl 2 and
in the oxide MgO, two electrons are transferred to form an Mg 2+ ion. Electrons in the next-to-last
shell may also be transferred. For example, iron has two outer shell electrons, but may form either
Fe 2+ or Fe 3+ ions. Many common salts, oxides, and other solids have bonds that are mostly or
partially ionic. These materials tend to be hard and brittle.
Covalent bonding involves the sharing of electrons and occurs where the outer shells are half
full or more than half full. The shared electrons can be thought of as allowing both atoms involved to
have stable outer shells of eight (or two) electrons. For example, two hydrogen atoms each share an
electron with an oxygen atom to make water, H 2 O, or two chlorine atoms share one electron to form
the diatomic molecule Cl 2 . The tight covalent bonds make such simple molecules relatively indepen-
dent of one another, so that collections of them tend to form liquids or gases at ambient temperatures.
Metallic bonding is responsible for the usually solid form of metals and alloys. For metals, the
outer shell of electrons is in most cases less than half full; each atom donates its outer shell electrons