Page 45 - Mechanical Behavior of Materials
P. 45
44 Chapter 2 Structure and Deformation in Materials
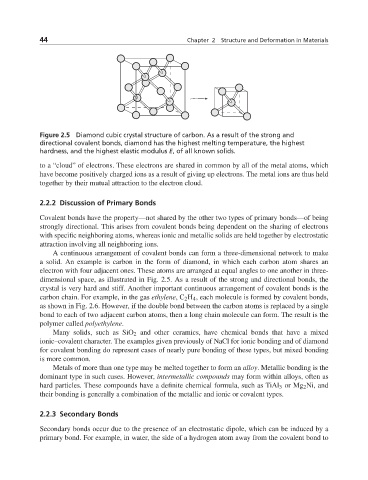
Figure 2.5 Diamond cubic crystal structure of carbon. As a result of the strong and
directional covalent bonds, diamond has the highest melting temperature, the highest
hardness, and the highest elastic modulus E, of all known solids.
to a “cloud” of electrons. These electrons are shared in common by all of the metal atoms, which
have become positively charged ions as a result of giving up electrons. The metal ions are thus held
together by their mutual attraction to the electron cloud.
2.2.2 Discussion of Primary Bonds
Covalent bonds have the property—not shared by the other two types of primary bonds—of being
strongly directional. This arises from covalent bonds being dependent on the sharing of electrons
with specific neighboring atoms, whereas ionic and metallic solids are held together by electrostatic
attraction involving all neighboring ions.
A continuous arrangement of covalent bonds can form a three-dimensional network to make
a solid. An example is carbon in the form of diamond, in which each carbon atom shares an
electron with four adjacent ones. These atoms are arranged at equal angles to one another in three-
dimensional space, as illustrated in Fig. 2.5. As a result of the strong and directional bonds, the
crystal is very hard and stiff. Another important continuous arrangement of covalent bonds is the
carbon chain. For example, in the gas ethylene,C 2 H 4 , each molecule is formed by covalent bonds,
as shown in Fig. 2.6. However, if the double bond between the carbon atoms is replaced by a single
bond to each of two adjacent carbon atoms, then a long chain molecule can form. The result is the
polymer called polyethylene.
Many solids, such as SiO 2 and other ceramics, have chemical bonds that have a mixed
ionic–covalent character. The examples given previously of NaCl for ionic bonding and of diamond
for covalent bonding do represent cases of nearly pure bonding of these types, but mixed bonding
is more common.
Metals of more than one type may be melted together to form an alloy. Metallic bonding is the
dominant type in such cases. However, intermetallic compounds may form within alloys, often as
hard particles. These compounds have a definite chemical formula, such as TiAl 3 or Mg 2 Ni, and
their bonding is generally a combination of the metallic and ionic or covalent types.
2.2.3 Secondary Bonds
Secondary bonds occur due to the presence of an electrostatic dipole, which can be induced by a
primary bond. For example, in water, the side of a hydrogen atom away from the covalent bond to