Page 196 - Membranes for Industrial Wastewater Recovery and Re-Use
P. 196
Industrial waters 165
Water is unique within all the pharmacopoeias in that it is the only material
commonly used by pharmaceutical companies which not only has quality
standards defined but production methods are also defined. Two main water
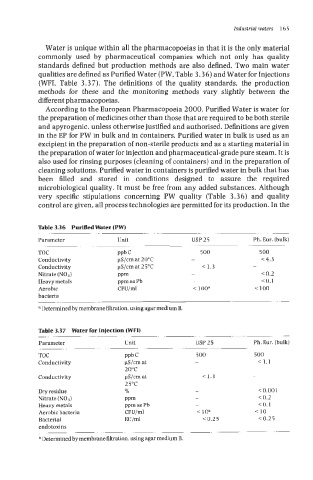
qualities are defined as Purified Water (PW, Table 3.36) and Water for Injections
(WFI, Table 3.37). The definitions of the quality standards, the production
methods for these and the monitoring methods vary slightly between the
different pharmacopoeias.
According to the European Pharmacopoeia 2000, Purified Water is water for
the preparation of medicines other than those that are required to be both sterile
and apyrogenic, unless otherwise justified and authorised. Definitions are given
in the EP for PW in bulk and in containers. Purified water in bulk is used as an
excipient in the preparation of non-sterile products and as a starting material in
the preparation of water for injection and pharmaceutical-grade pure steam. It is
also used for rinsing purposes (cleaning of containers) and in the preparation of
cleaning solutions. Purified water in containers is purified water in bulk that has
been filled and stored in conditions designed to assure the required
microbiological quality. It must be free from any added substances. Although
very specific stipulations concerning PW quality (Table 3.36) and quality
control are given, all process technologies are permitted for its production. In the
Table 3.36 PurifiedWater (PW)
Parameter Unit USP25 Ph. Eur. (bulk)
TOC ppb C 500 500
Conductivity pS/cm at 20°C - < 4.3
Conductivity FS/cm at 2 5°C < 1.3 -
Nitrate (N03) PPm - < 0.2
Heavy metals ppm as Pb - <0.1
Aerobic CFU/ml < 100a < 100
bacteria
a Determined by membrane filtration, using agar medium B.
Table 3.37 Water for Injection (WFI)
Parameter Unit USP25 Ph. Eur. (bulk)
TOC PPb c 500 500
Conductivity pS/cm at - < 1.1
20°C
Conductivity pS/cm at < 1.3 -
25°C
Dry residue Yo - < 0.001
Nitrate (NO3) PPm - < 0.2
Heavy metals ppm as Pb - <0.1
Aerobic bacteria CFU/ml <loa <I0
Bacterial EU/ml <0.25 10.25
endotoxins
a Determined by membrane filtration, using agar medium B.