Page 58 - Membranes for Industrial Wastewater Recovery and Re-Use
P. 58
38 Membranes for Industrial Wastewater Recovery and Re-use
F
concentrate
diluatc C1
7
OH
anion-exchanging anion-e:.,..ianging
membrane
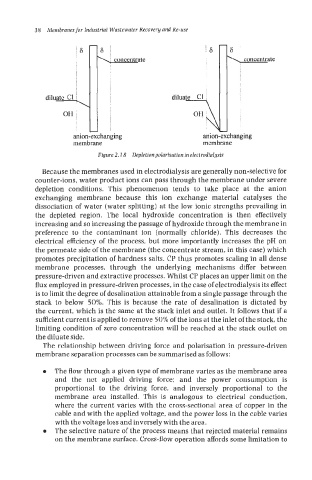
Figure 2.1 8 Depletion polarisation in electrodialysis
Because the membranes used in electrodialysis are generally non-selective for
counter-ions, water product ions can pass through the membrane under severe
depletion conditions. This phenomenon tends to take place at the anion
exchanging membrane because this ion exchange material catalyses the
dissociation of water (water splitting) at the low ionic strengths prevailing in
the depleted region. The local hydroxide concentration is then effectively
increasing and so increasing the passage of hydroxide through the membrane in
preference to the contaminant ion (normally chloride). This decreases thc
electrical efficiency of the process, but more importantly increases the pH on
the permeate side of the membrane (the concentrate stream, in this case) which
promotes precipitation of hardness salts. CP thus promotes scaling in all dense
membrane processes, through the underlying mechanisms differ between
pressure-driven and extractive processes. Whilst CP places an upper limit on the
flux employed in pressure-driven processes, in the case of electrodialysis its effect
is to limit the degree of desalination attainable from a single passage through the
stack to below 50%. This is because the rate of desalination is dictated by
the current, which is the same at the stack inlet and outlet. It follows that if a
sufficient current is applied to remove 50% of the ions at the inlet of the stack, the
limiting condition of zero concentration will be reached at the stack outlet on
the diluate side.
The relationship between driving force and polarisation in pressure-driven
membrane separation processes can be summarised as follows:
0 The flow through a given type of membrane varies as the membrane area
and the net applied driving force: and the power consumption is
proportional to the driving force, and inversely proportional to the
membrane area installed. This is analogous to electrical conduction,
where the current varies with the cross-sectional area of copper in the
cable and with the applied voltage, and the power loss in the cable varies
with the voltage loss and inversely with the area.
0 The selective nature of the process means that rejected material remains
on the membrane surface. Cross-flow operation affords some limitation to