Page 56 - Membranes for Industrial Wastewater Recovery and Re-Use
P. 56
3 6 Membranes for Industrial Wastewater Recovery and Re-use
In the case of a dead-end filtration process, the resistance increases according
to the thickness of the cake formed on the membrane, which would be expected to
be proportional to the total volume of filtrate passed. For cross-flow processes,
this deposition continues until the adhesive forces binding the cake to the
membrane are balanced by the scouring forces of the liquid passing over
the membrane. All other things being equal, a cross-flow filtration process would
be expected to attain steady-state conditions. In practice, only pseudo-steady-
state (or stabilised) conditions are attained due to the unavoidable deposition or
adsorption of fouling material.
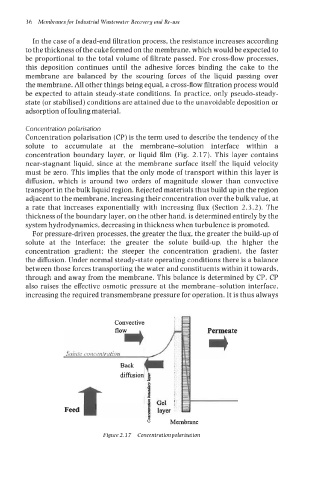
Concentration polarisation
Concentration polarisation (CP) is the term used to describe the tendency of the
solute to accumulate at the membrane-solution interface within a
concentration boundary layer, or liquid film (Fig. 2.17). This layer contains
near-stagnant liquid, since at the membrane surface itself the liquid velocity
must be zero. This implies that the only mode of transport within this layer is
diffusion, which is around two orders of magnitude slower than convective
transport in the bulk liquid region. Rejected materials thus build up in the region
adjacent to the membrane, increasing their concentration over the bulk value, at
a rate that increases exponentially with increasing flux (Section 2.3.2). The
thickness of the boundary layer, on the other hand, is determined entirely by the
system hydrodynamics, decreasing in thickness when turbulence is promoted.
For pressure-driven processes, the greater the flux, the greater the build-up of
solute at the interface: the greater the solute build-up, the higher the
concentration gradient: the steeper the concentration gradient, the faster
the diffusion. Under normal steady-state operating conditions there is a balance
between those forces transporting the water and constituents within it towards,
through and away from the membrane. This balance is determined by CP. CP
also raises the effective osmotic pressure at the membrane-solution interface,
increasing the required transmembrane pressure for operation. It is thus always
convectiv4
Permeate
1
Membrane
Figure2.17 Concentrationpolarisation