Page 237 - Modeling of Chemical Kinetics and Reactor Design
P. 237
Reaction Rate Expression 207
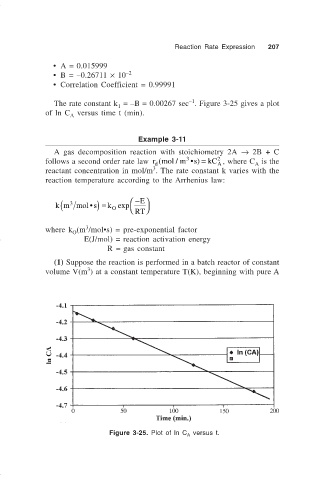
• A = 0.015999
• B = –0.26711 × 10 –2
• Correlation Coefficient = 0.99991
–1
The rate constant k = –B = 0.00267 sec . Figure 3-25 gives a plot
1
of ln C versus time t (min).
A
Example 3-11
A gas decomposition reaction with stoichiometry 2A → 2B + C
2
follows a second order rate law r mol m( / 3 s • ) = kC , where C is the
d
A
A
3
reactant concentration in mol/m . The rate constant k varies with the
reaction temperature according to the Arrhenius law:
(
k m mol s) = k exp − E
3
•
O
RT
3
where k (m /mol•s) = pre-exponential factor
O
E(J/mol) = reaction activation energy
R = gas constant
(1) Suppose the reaction is performed in a batch reactor of constant
3
volume V(m ) at a constant temperature T(K), beginning with pure A
Figure 3-25. Plot of ln C versus t.
A