Page 234 - Modeling of Chemical Kinetics and Reactor Design
P. 234
204 Modeling of Chemical Kinetics and Reactor Design
1
− 1 = kC BO t (3-306)
1− X B
1
− 1 = kC BO t (3-307)
C B C BO
The rate constant k can be calculated from Equation 3-307 as:
k = 1 1 −1 (3-308)
C BO B C BO
t C
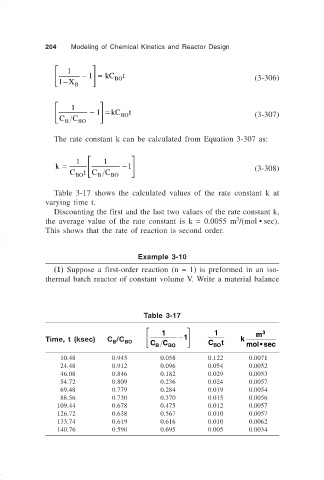
Table 3-17 shows the calculated values of the rate constant k at
varying time t.
Discounting the first and the last two values of the rate constant k,
3
the average value of the rate constant is k = 0.0055 m /(mol • sec).
This shows that the rate of reaction is second order.
Example 3-10
(1) Suppose a first-order reaction (n = 1) is preformed in an iso-
thermal batch reactor of constant volume V. Write a material balance
Table 3-17
1 1 m 3
Time, t (ksec) C /C BO − 1 k
C B C BO C BO t mol• sec
B
10.48 0.945 0.058 0.122 0.0071
24.48 0.912 0.096 0.054 0.0052
46.08 0.846 0.182 0.029 0.0053
54.72 0.809 0.236 0.024 0.0057
69.48 0.779 0.284 0.019 0.0054
88.56 0.730 0.370 0.015 0.0056
109.44 0.678 0.475 0.012 0.0057
126.72 0.638 0.567 0.010 0.0057
133.74 0.619 0.616 0.010 0.0062
140.76 0.590 0.695 0.005 0.0034