Page 246 - Modeling of Chemical Kinetics and Reactor Design
P. 246
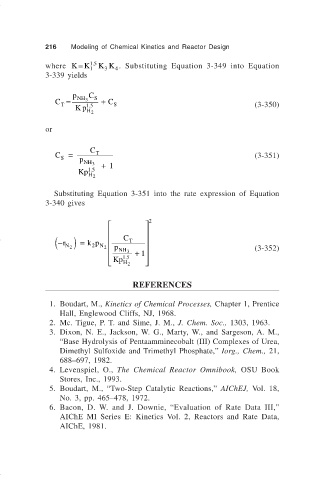
216 Modeling of Chemical Kinetics and Reactor Design
where KK= 15 K K . Substituting Equation 3-349 into Equation
.
4
1
3
3-339 yields
p C S
C = NH 3 + C
T 15 S (3-350)
.
Kp
H 2
or
C S = p C T (3-351)
NH 3 + 1
.
Kp 15
H 2
Substituting Equation 3-351 into the rate expression of Equation
3-340 gives
2
− ( r ) = k p C T
p NH 3 (3-352)
N 2 2 N 2
15 . + 1
Kp H 2
REFERENCES
1. Boudart, M., Kinetics of Chemical Processes, Chapter 1, Prentice
Hall, Englewood Cliffs, NJ, 1968.
2. Mc. Tigue, P. T. and Sime, J. M., J. Chem. Soc., 1303, 1963.
3. Dixon, N. E., Jackson, W. G., Marty, W., and Sargeson, A. M.,
“Base Hydrolysis of Pentaamminecobalt (III) Complexes of Urea,
Dimethyl Sulfoxide and Trimethyl Phosphate,” Iorg., Chem., 21,
688–697, 1982.
4. Levenspiel, O., The Chemical Reactor Omnibook, OSU Book
Stores, Inc., 1993.
5. Boudart, M., “Two-Step Catalytic Reactions,” AIChEJ, Vol. 18,
No. 3, pp. 465–478, 1972.
6. Bacon, D. W. and J. Downie, “Evaluation of Rate Data III,”
AIChE MI Series E: Kinetics Vol. 2, Reactors and Rate Data,
AIChE, 1981.