Page 286 - Modeling of Chemical Kinetics and Reactor Design
P. 286
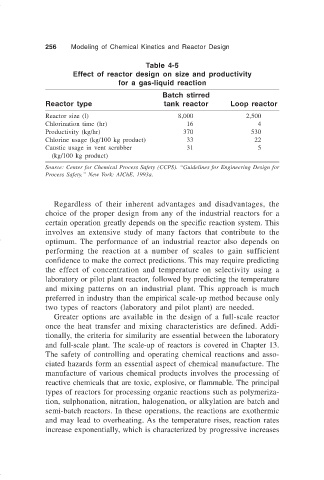
256 Modeling of Chemical Kinetics and Reactor Design
Table 4-5
Effect of reactor design on size and productivity
for a gas-liquid reaction
Batch stirred
Reactor type tank reactor Loop reactor
Reactor size (l) 8,000 2,500
Chlorination time (hr) 16 4
Productivity (kg/hr) 370 530
Chlorine usage (kg/100 kg product) 33 22
Caustic usage in vent scrubber 31 5
(kg/100 kg product)
Source: Center for Chemical Process Safety (CCPS). “Guidelines for Engineering Design for
Process Safety.” New York: AIChE, 1993a.
Regardless of their inherent advantages and disadvantages, the
choice of the proper design from any of the industrial reactors for a
certain operation greatly depends on the specific reaction system. This
involves an extensive study of many factors that contribute to the
optimum. The performance of an industrial reactor also depends on
performing the reaction at a number of scales to gain sufficient
confidence to make the correct predictions. This may require predicting
the effect of concentration and temperature on selectivity using a
laboratory or pilot plant reactor, followed by predicting the temperature
and mixing patterns on an industrial plant. This approach is much
preferred in industry than the empirical scale-up method because only
two types of reactors (laboratory and pilot plant) are needed.
Greater options are available in the design of a full-scale reactor
once the heat transfer and mixing characteristics are defined. Addi-
tionally, the criteria for similarity are essential between the laboratory
and full-scale plant. The scale-up of reactors is covered in Chapter 13.
The safety of controlling and operating chemical reactions and asso-
ciated hazards form an essential aspect of chemical manufacture. The
manufacture of various chemical products involves the processing of
reactive chemicals that are toxic, explosive, or flammable. The principal
types of reactors for processing organic reactions such as polymeriza-
tion, sulphonation, nitration, halogenation, or alkylation are batch and
semi-batch reactors. In these operations, the reactions are exothermic
and may lead to overheating. As the temperature rises, reaction rates
increase exponentially, which is characterized by progressive increases