Page 176 - Modelling in Transport Phenomena A Conceptual Approach
P. 176
156 CHAPTER 6. STEADY-STATE MACROSCOPIC BALANCES
-8.673 -50.632 -2.284 ][g=[7i]
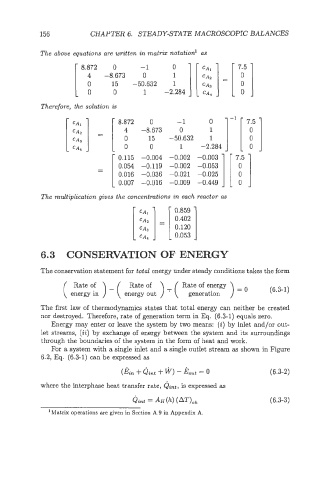
The above equations are written in matrix notation1 as
8.872 0 -1 0
4 15 0 1
0
1
Therefore, the solution is
8.872 0 -1 0
0
4 -8.673 -50.632 1
0
15
0.054 -0.119 -0.002 -0.0531 [
1
-2.284
0
0
0.115 -0.004 -0.002 -0.003
0.016 -0.036 -0.021 -0.025
[ :::I 0.859
0.007 -0.016 -0.009 -0.449
The multiplication gives the concentrations in each reactor as
CAI
0.053
CAS = [ 0.402 0.1201
6.3 CONSERVATION OF ENERGY
The conservation statement for total energy under steady conditions takes the form
( Rate of ) - ( Rate of ) + ( Rate of energy
energy in energy out generation ) =O (6.3-1)
The first law of thermodynamics states that total energy can neither be created
nor destroyed. Therefore, rate of generation term in Eq. (6.3-1) equals zero.
Energy may enter or leave the system by two means: (i) by inlet and/or out-
let streams, (ii) by exchange of energy between the system and its surroundings
through the boundaries of the system in the form of heat and work.
For a system with a single inlet and a single outlet stream as shown in Figure
6.2, Eq. (6.3-1) can be expressed as
where the interphase heat transfer rate, Qint, is expressed as
Qint = AH(^) (AT),, (6.3-3)
'Matrix operations are given in Section A.9 in Appendix A.