Page 181 - Modelling in Transport Phenomena A Conceptual Approach
P. 181
6.3. CONSERVATION OF ENERGY 161
6.3.1.2 Constant pressure with phase change
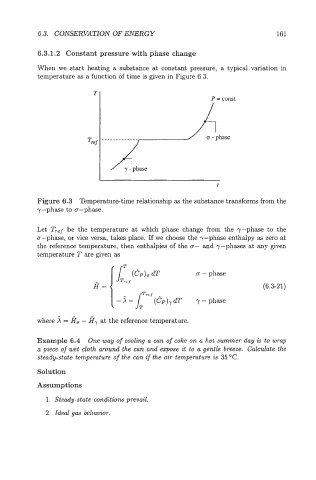
When we start heating a substance at constant pressure, a typical variation in
temperature as a function of time is given in Figure 6.3.
T
P = const
=rer
t
Figure 6.3 Temperaturetime relationship as the substance transforms from the
y-phase to cr-phase.
Let T,,f be the temperature at which phase change from the y-phase to the
a-phase, or vice versa, takes place. If we choose the y-phase enthalpy as zero at
the reference temperature, then enthalpies of the cr- and 7-phases at any given
temperature T are given as
(6.3-21)
where 5 = HT, - HT at the reference temperature.
Example 6.4 One way of cooling a can of coke on a hot summer day is to wap
a piece of wet cloth around the can and eqose it to a gentle breeze. Calculate the
steady-state temperature of the can if the air temperature is 35°C.
Solution
Assumptions
1. Steady-state conditions prevail.
2. Ideal gas behavior.