Page 185 - Modelling in Transport Phenomena A Conceptual Approach
P. 185
6.3. CONSERVATION OF ENERGY 165
6.3.1.3 Variable pressure and no phase change
Enthalpy of an ideal gas is dependent only on temperature and is expressed by Eq.
(6.3-18). Therefore, in problems involving ideal gases, variation in pressure has no
effect on the enthalpy change. In the case of incompressible fluids, Eq. (6.3-12)
reduces to
.-T
(6.3-22)
in which the enthalpy is taken zero at the reference temperature and pressure. At
low and moderate pressures, the second term on the right-side of Eq. (6.3-22) is
usually considered negligible.
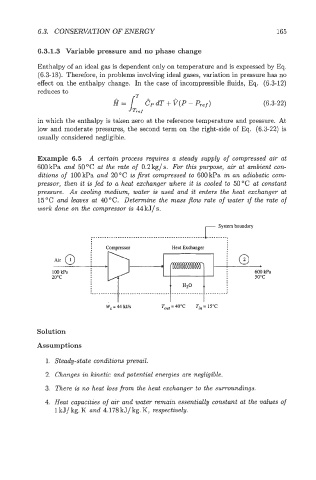
Example 6.5 A certain process reqzlires a steady supply of compressed air at
6OOkPa and 50°C at the rate of 0.2kg/s. For this purpose, air at ambient con-
ditions of 100 kPa and 20 "C is first compressed to 600 kPa in an adiabatic com-
pressor, then it is fed to a heat exchanger where it is cooled to 50°C at constant
pressure. As cooling medium, water is used and it enters the heat exchanger at
15°C and leaves at 40°C. Determine the mass flow rate of water if the rate of
work done on the compressor is 44 kJ/ s.
r System boundary
*
100 kPa 600 kPa
20°C 50°C
H20
"
"
._---------- ................................................ 1
Solution
Assumptions
1. Steady-state conditions prevail.
2. Changes in kinetic and potential energies are negligible.
3. There is no heat loss from the heat exchanger to the surroundings.
4. Heat capacities of air and water remain essentially constant at the values of
1 kJ/ kg. K and 4.178 kJ/ kg. K, respectively.