Page 191 - Modelling in Transport Phenomena A Conceptual Approach
P. 191
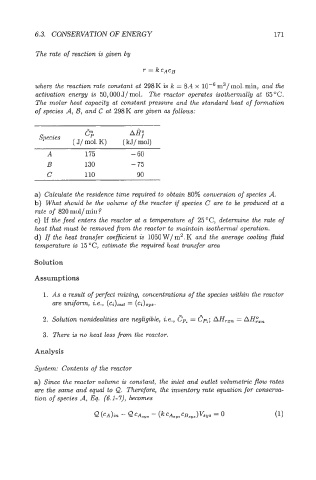
6.3. CONSERVATION OF ENERGY 171
The rate of reaction is given by
where the reaction rate constant at 298 K is k = 8.4 x m3/ mol. min, and the
activation energy is 50,000 J/ mol. The reactor operates isothermally at 65 "C.
The molar heat capacity at constant pressure and the standard heat of formation
of species A, 23, and C at 298K are given as follows:
ep AH;
Species
( J/ mol. K) ( kJ/ mol)
A 175 - 60
B 130 - 75
C 110 - 90
a) Calculate the residence time required to obtain 80% conversion of species A.
b) What should be the volume of the reactor if species C are to be produced at a
rate of 820 mol/ min?
c) If the feed enters the reactor at a temperature of 25"C, determine the rate of
heat that must be removed from the reactor to maintain isothermal operation.
K
d) If the heat transfer coeficient is IO50 W/ m2, and the average cooling fluid
temperature is 15°C) estimate the required heat transfer area
Solution
Assumptions
1. As a result of perfect mixing, concentrations of the species within the reactor
are uniform, i.e., (~),,t = (G)~~~.
2. Solution nonidealities are negligible, i.e., cp, = cpi; AH,.,, = AH&,
3. There is no heat loss from the reactor.
Analysis
System: Contents of the reactor
a) Since the reactor volume is constant, the inlet and outlet volumetric flow rates
are the same and equal to &. Therefore, the inventory rate equation for comerua-
tion of species A, Eq. (6.1-7)) becomes
& (C~)in - &CA,,, - (kCAaYaCBsya)%ys = 0 (1)