Page 321 - Modelling in Transport Phenomena A Conceptual Approach
P. 321
8.5. MASS TRANSPORT WITH CONVECTION 301
When A ---t 0, this means that the rate of diffusion is much larger than the rate
of reaction. The Taylor series expansion of q in terms of A gives
17
1
2
17 = 1 - - A2 + - A4 - - + ... (8.4-76)
A6
3 15 315
Therefore, q approaches unity as A + 0, indicating that the entire surface is
exposed to a reactant. On the other hand, large values of A corresponds to cases
in which diffusion rate is very slow and the surface reaction is very rapid. Under
these conditions the effectiveness factor becomes
1
q=- (8.4- 77)
A
As A + OO,~ approaches zero. This implies that a good part of the catalyst surface
is starved for a reactant and hence not effective.
8.5 MASS TFUNSPORT WITH
CONVECTION
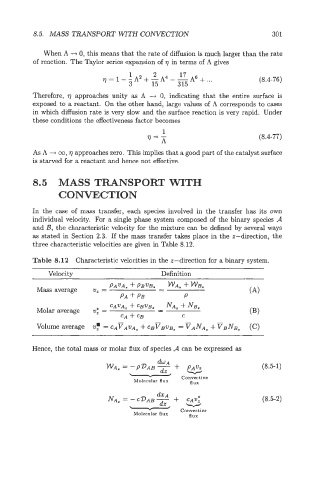
In the case of mass transfer, each species involved in the transfer has its own
individual velocity. For a single phase system composed of the binary species A
and 23, the characteristic velocity for the mixture can be defined by several ways
as stated in Section 2.3. If the mas transfer takes place in the z-direction, the
three characteristic velocities are given in Table 8.12.
Table 8.12 Characteristic velocities in the z-direction for a binary system.
Velocity Definition
-
PAVA= + PBVB. - WA, + WB,
Mass average v, =
PA + PB P
-
Hence, the total mass or molar flux of species A can be expressed as
&A
WA. = - P~AB + PAv% (8.5-1)
v
-
Convective
Molecular flux
flux
NA, = - CDAB - v (8.5-2)
dXA
-k
CAv:
dz
Molecular flux Convective
flux