Page 218 - Modern Analytical Chemistry
P. 218
1400-CH07 9/8/99 4:03 PM Page 201
Chapter 7 Obtaining and Preparing Samples for Analysis 201
7 3
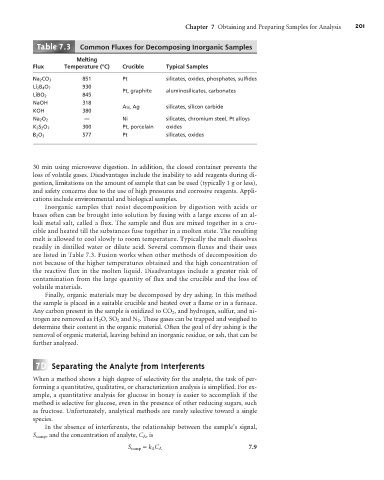
Table . Common Fluxes for Decomposing Inorganic Samples
Melting
Flux Temperature (°C) Crucible Typical Samples
Na 2 CO 3 851 Pt silicates, oxides, phosphates, sulfides
Li 2 B 4 O 7 930
Pt, graphite aluminosilicates, carbonates
LiBO 2 845
NaOH 318
Au, Ag silicates, silicon carbide
KOH 380
Na 2 O 2 — Ni silicates, chromium steel, Pt alloys
K 2 S 2 O 7 300 Pt, porcelain oxides
B 2 O 3 577 Pt silicates, oxides
30 min using microwave digestion. In addition, the closed container prevents the
loss of volatile gases. Disadvantages include the inability to add reagents during di-
gestion, limitations on the amount of sample that can be used (typically 1 g or less),
and safety concerns due to the use of high pressures and corrosive reagents. Appli-
cations include environmental and biological samples.
Inorganic samples that resist decomposition by digestion with acids or
bases often can be brought into solution by fusing with a large excess of an al-
kali metal salt, called a flux. The sample and flux are mixed together in a cru-
cible and heated till the substances fuse together in a molten state. The resulting
melt is allowed to cool slowly to room temperature. Typically the melt dissolves
readily in distilled water or dilute acid. Several common fluxes and their uses
are listed in Table 7.3. Fusion works when other methods of decomposition do
not because of the higher temperatures obtained and the high concentration of
the reactive flux in the molten liquid. Disadvantages include a greater risk of
contamination from the large quantity of flux and the crucible and the loss of
volatile materials.
Finally, organic materials may be decomposed by dry ashing. In this method
the sample is placed in a suitable crucible and heated over a flame or in a furnace.
Any carbon present in the sample is oxidized to CO 2, and hydrogen, sulfur, and ni-
trogen are removed as H 2O, SO 2 and N 2. These gases can be trapped and weighed to
determine their content in the organic material. Often the goal of dry ashing is the
removal of organic material, leaving behind an inorganic residue, or ash, that can be
further analyzed.
7 D Separating the Analyte from Interferents
When a method shows a high degree of selectivity for the analyte, the task of per-
forming a quantitative, qualitative, or characterization analysis is simplified. For ex-
ample, a quantitative analysis for glucose in honey is easier to accomplish if the
method is selective for glucose, even in the presence of other reducing sugars, such
as fructose. Unfortunately, analytical methods are rarely selective toward a single
species.
In the absence of interferents, the relationship between the sample’s signal,
S samp , and the concentration of analyte, C A , is
7.9
S samp = k A C A