Page 30 - Modern Analytical Chemistry
P. 30
1400-CH02 9/8/99 3:47 PM Page 13
Chapter 2 Basic Tools of Analytical Chemistry 13
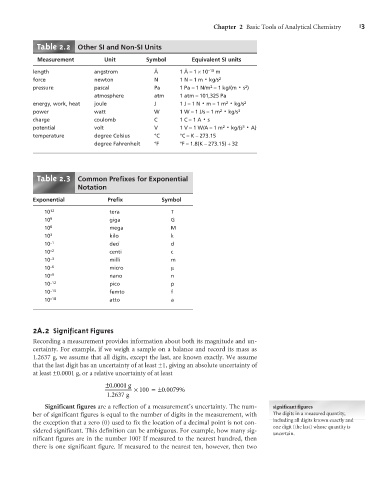
Table 2.2 Other SI and Non-SI Units
Measurement Unit Symbol Equivalent SI units
length angstrom Å 1 Å =1 ´10 –10 m
×
force newton N 1 N =1 m kg/s 2
×
2
2
pressure pascal Pa 1 Pa =1 N/m =1 kg/(m s )
atmosphere atm 1 atm =101,325 Pa
×
kg/s
energy, work, heat joule J 1 J =1 N m =1 m 2 × 2
kg/s
power watt W 1 W =1 J/s =1 m 2 × 3
×
charge coulomb C 1 C =1 A s
3
potential volt V 1 V =1 W/A =1 m 2 × ×
kg/(s
A)
temperature degree Celsius °C °C =K – 273.15
degree Fahrenheit °F °F =1.8(K – 273.15) +32
Table 2.3 Common Prefixes for Exponential
Notation
Exponential Prefix Symbol
10 12 tera T
10 9 giga G
10 6 mega M
10 3 kilo k
10 –1 deci d
10 –2 centi c
10 –3 milli m
10 –6 micro m
10 –9 nano n
10 –12 pico p
10 –15 femto f
10 –18 atto a
2A.2 Significant Figures
Recording a measurement provides information about both its magnitude and un-
certainty. For example, if we weigh a sample on a balance and record its mass as
1.2637 g, we assume that all digits, except the last, are known exactly. We assume
that the last digit has an uncertainty of at least ±1, giving an absolute uncertainty of
at least ±0.0001 g, or a relative uncertainty of at least
±0 0001 g
.
´100 = ±0079%
0
.
1 2637 g
.
Significant figures are a reflection of a measurement’s uncertainty. The num- significant figures
ber of significant figures is equal to the number of digits in the measurement, with The digits in a measured quantity,
the exception that a zero (0) used to fix the location of a decimal point is not con- including all digits known exactly and
one digit (the last) whose quantity is
sidered significant. This definition can be ambiguous. For example, how many sig-
uncertain.
nificant figures are in the number 100? If measured to the nearest hundred, then
there is one significant figure. If measured to the nearest ten, however, then two