Page 363 - Modern Analytical Chemistry
P. 363
1400-CH09 9/9/99 2:13 PM Page 346
346 Modern Analytical Chemistry
Organic Analysis Redox titrimetric methods also are used for the analysis of or-
ganic analytes. One important example is the determination of the chemical oxygen
demand (COD) in natural waters and wastewaters. The COD provides a measure of
the quantity of oxygen necessary to completely oxidize all the organic matter in a
sample to CO 2 and H 2 O. No attempt is made to correct for organic matter that can-
not be decomposed biologically or for which the decomposition kinetics are very
slow. Thus, the COD always overestimates a sample’s true oxygen demand. The de-
termination of COD is particularly important in managing industrial wastewater
treatment facilities where it is used to monitor the release of organic-rich wastes
into municipal sewer systems or the environment.
The COD is determined by refluxing the sample in the presence of excess
K 2 Cr 2 O 7 , which serves as the oxidizing agent. The solution is acidified with H 2 SO 4 ,
and Ag 2 SO 4 is added as a catalyst to speed the oxidation of low-molecular-weight
fatty acids. Mercuric sulfate, HgSO 4 , is added to complex any chloride that is pres-
+
ent, thus preventing the precipitation of the Ag catalyst as AgCl. Under these con-
ditions, the efficiency for oxidizing organic matter is 95–100%. After refluxing for
2–
2h, the solution is cooled to room temperature, and the excess Cr 2 O 7 is deter-
mined by a back titration, using ferrous ammonium sulfate as the titrant and fer-
roin as the indicator. Since it is difficult to completely remove all traces of organic
matter from the reagents, a blank titration must be performed. The difference in the
amount of ferrous ammonium sulfate needed to titrate the blank and the sample is
proportional to the COD.
Iodine has been used as an oxidizing titrant for a number of compounds of
2– –
pharmaceutical interest. Earlier we noted that the reaction of S 2 O 3 with I 3 pro-
2–
duces the tetrathionate ion, S 4 O 6 . The tetrathionate ion is actually a dimer consist-
ing of two thiosulfate ions connected through a disulfide (-S-S-) linkage. In the
–
same fashion, I 3 can be used to titrate mercaptans of the general formula RSH,
forming the dimer RSSR as a product. The amino acid cysteine also can be titrated
–
with I 3 . The product of this titration is cystine, which is a dimer of cysteine. Triio-
dide also can be used for the analysis of ascorbic acid (vitamin C) by oxidizing the
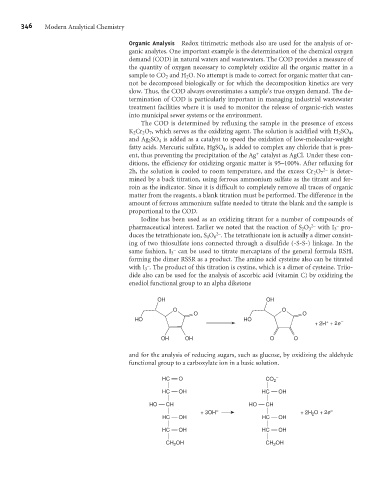
enediol functional group to an alpha diketone
OH OH
O O
O O
HO HO
+
+ 2H + 2e –
OH OH O O
and for the analysis of reducing sugars, such as glucose, by oxidizing the aldehyde
functional group to a carboxylate ion in a basic solution.
HC O CO 2 –
HC OH HC OH
HO CH HO CH
+ 3OH – + 2H 2 O + 2e –
HC OH HC OH
HC OH HC OH
CH OH CH OH
2
2