Page 37 - Modern Analytical Chemistry
P. 37
1400-CH02 9/8/99 3:48 PM Page 20
20 Modern Analytical Chemistry
0.12 14
+
[H ]
pH 12
0.10
10
0.08
[H + ] (M) 0.06 8 pH
6
0.04
4
0.02
2
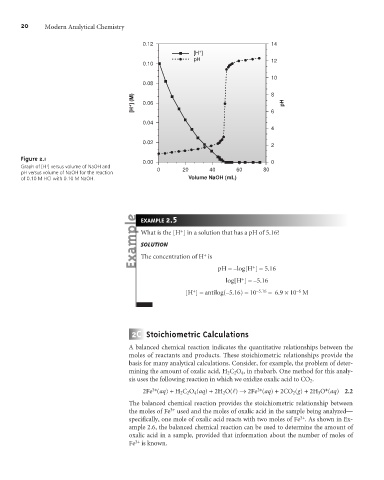
Figure 2.1
0.00 0
+
Graph of [H ] versus volume of NaOH and
pH versus volume of NaOH for the reaction 0 20 40 60 80
of 0.10 M HCl with 0.10 M NaOH. Volume NaOH (mL)
EXAMPLE 2. 5
+
What is the [H ] in a solution that has a pH of 5.16?
SOLUTION
+
The concentration of H is
pH =–log[H ] =5.16
+
+
log[H ] =–5.16
–6
[H ] =antilog(–5.16) =10 –5.16 = 6.9 ´10 M
+
2C Stoichiometric Calculations
A balanced chemical reaction indicates the quantitative relationships between the
moles of reactants and products. These stoichiometric relationships provide the
basis for many analytical calculations. Consider, for example, the problem of deter-
mining the amount of oxalic acid, H 2 C 2 O 4 , in rhubarb. One method for this analy-
sis uses the following reaction in which we oxidize oxalic acid to CO 2 .
+
2+
3+
2Fe (aq) +H 2 C 2 O 4 (aq) +2H 2 O(l) ® 2Fe (aq) +2CO 2 (g) +2H 3 O (aq) 2.2
The balanced chemical reaction provides the stoichiometric relationship between
the moles of Fe used and the moles of oxalic acid in the sample being analyzed—
3+
3+
specifically, one mole of oxalic acid reacts with two moles of Fe . As shown in Ex-
ample 2.6, the balanced chemical reaction can be used to determine the amount of
oxalic acid in a sample, provided that information about the number of moles of
3+
Fe is known.