Page 378 - Modern Analytical Chemistry
P. 378
1400-CH09 9/9/99 2:14 PM Page 361
Chapter 9 Titrimetric Methods of Analysis 361
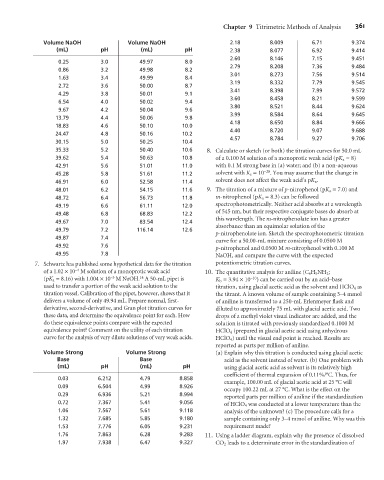
Volume NaOH Volume NaOH 2.18 8.009 6.71 9.374
(mL) pH (mL) pH 2.38 8.077 6.92 9.414
2.60 8.146 7.15 9.451
0.25 3.0 49.97 8.0
2.79 8.208 7.36 9.484
0.86 3.2 49.98 8.2
3.01 8.273 7.56 9.514
1.63 3.4 49.99 8.4
3.19 8.332 7.79 9.545
2.72 3.6 50.00 8.7
3.41 8.398 7.99 9.572
4.29 3.8 50.01 9.1
3.60 8.458 8.21 9.599
6.54 4.0 50.02 9.4
3.80 8.521 8.44 9.624
9.67 4.2 50.04 9.6
3.99 8.584 8.64 9.645
13.79 4.4 50.06 9.8
4.18 8.650 8.84 9.666
18.83 4.6 50.10 10.0
4.40 8.720 9.07 9.688
24.47 4.8 50.16 10.2
4.57 8.784 9.27 9.706
30.15 5.0 50.25 10.4
35.33 5.2 50.40 10.6 8. Calculate or sketch (or both) the titration curves for 50.0 mL
39.62 5.4 50.63 10.8 of a 0.100 M solution of a monoprotic weak acid (pK a =8)
42.91 5.6 51.01 11.0 with 0.1 M strong base in (a) water; and (b) a non-aqueous
45.28 5.8 51.61 11.2 solvent with K s =10 –20 . You may assume that the change in
46.91 6.0 52.58 11.4 solvent does not affect the weak acid’s pK a .
48.01 6.2 54.15 11.6 9. The titration of a mixture of p-nitrophenol (pK a = 7.0) and
48.72 6.4 56.73 11.8 m-nitrophenol (pK a = 8.3) can be followed
49.19 6.6 61.11 12.0 spectrophotometrically. Neither acid absorbs at a wavelength
of 545 nm, but their respective conjugate bases do absorb at
49.48 6.8 68.83 12.2
this wavelength. The m-nitrophenolate ion has a greater
49.67 7.0 83.54 12.4
absorbance than an equimolar solution of the
49.79 7.2 116.14 12.6
p-nitrophenolate ion. Sketch the spectrophotometric titration
49.87 7.4
curve for a 50.00-mL mixture consisting of 0.0500 M
49.92 7.6 p-nitrophenol and 0.0500 M m-nitrophenol with 0.100 M
49.95 7.8 NaOH, and compare the curve with the expected
7. Schwartz has published some hypothetical data for the titration potentiometric titration curves.
–4
of a 1.02 ´10 M solution of a monoprotic weak acid 10. The quantitative analysis for aniline (C 6 H 5 NH 2 ;
14
–3
(pK a = 8.16) with 1.004 ´10 M NaOH. A 50-mL pipet is K b = 3.94 ´10 –10 ) can be carried out by an acid–base
used to transfer a portion of the weak acid solution to the titration, using glacial acetic acid as the solvent and HClO 4 as
titration vessel. Calibration of the pipet, however, shows that it the titrant. A known volume of sample containing 3–4 mmol
delivers a volume of only 49.94 mL. Prepare normal, first- of aniline is transferred to a 250-mL Erlenmeyer flask and
derivative, second-derivative, and Gran plot titration curves for diluted to approximately 75 mL with glacial acetic acid. Two
these data, and determine the equivalence point for each. How drops of a methyl violet visual indicator are added, and the
do these equivalence points compare with the expected solution is titrated with previously standardized 0.1000 M
equivalence point? Comment on the utility of each titration HClO 4 (prepared in glacial acetic acid using anhydrous
curve for the analysis of very dilute solutions of very weak acids. HClO 4) until the visual end point is reached. Results are
reported as parts per million of aniline.
Volume Strong Volume Strong (a) Explain why this titration is conducted using glacial acetic
Base Base acid as the solvent instead of water. (b) One problem with
(mL) pH (mL) pH using glacial acetic acid as solvent is its relatively high
coefficient of thermal expansion of 0.11%/°C. Thus, for
0.03 6.212 4.79 8.858
example, 100.00 mL of glacial acetic acid at 25 °C will
0.09 6.504 4.99 8.926
occupy 100.22 mL at 27 °C. What is the effect on the
0.29 6.936 5.21 8.994 reported parts per million of aniline if the standardization
0.72 7.367 5.41 9.056 of HClO 4 was conducted at a lower temperature than the
1.06 7.567 5.61 9.118 analysis of the unknown? (c) The procedure calls for a
1.32 7.685 5.85 9.180 sample containing only 3–4 mmol of aniline. Why was this
1.53 7.776 6.05 9.231 requirement made?
1.76 7.863 6.28 9.283 11. Using a ladder diagram, explain why the presence of dissolved
1.97 7.938 6.47 9.327 CO 2 leads to a determinate error in the standardization of