Page 15 - MODERN ASPECTS OF ELECTROCHEMISTRY
P. 15
Michael SpiroA
2
potential it acts simultaneouslyas an anode for the couple of lower Nernst
potential
→ Ox +ne -
Red 1 1 (1)
and as a cathode for the couple of higher Nernst potential
-
+ ne →Red
Ox 2 2 (2)
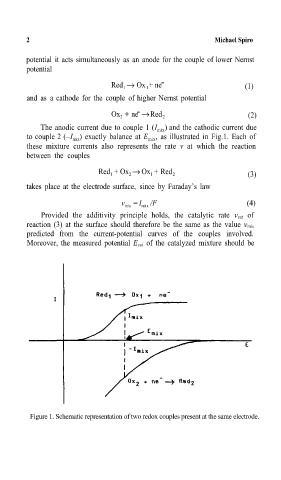
The anodic current due to couple 1 (I ) and the cathodic current due
mix
to couple 2 (–I ) exactly balance at E , as illustrated in Fig.1. Each of
mix
mix
these mixture currents also represents the rate v at which the reaction
between the couples
Red + Ox → Ox + Red 2 (3)
2
1
1
t¸es place at the electrode surface, since byFaraday’s law
v mix =I mix /F (4)
Provided the additivity principle holds, the catalytic rate v cat of
reaction (3) at the surface should therefore be the same as the value v mix
predicted from the current-potential curves of the couples involved.
Moreover, the measured potential E cat of the catalyzed mixture should be
Figure. Schematic representation of two redox couples present at the same electrode.=