Page 23 - MODERN ASPECTS OF ELECTROCHEMISTRY
P. 23
10
Michael SpiroA
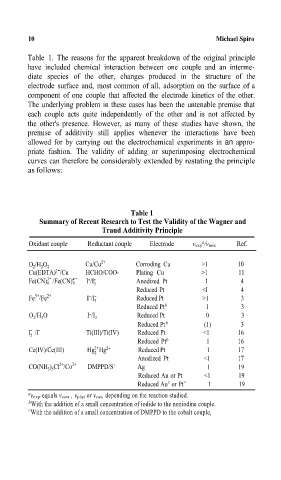
Tablp 1. The reasons for the apparent bre¸down of the original principle
have included chemical interaction between one couple and an interme-
diate species of the other, changes produced in the structure of the
electrode surface and, most common of all, adsorption on the surface of a
component of one couple that affected the electrode kinetics of the other.
The underlying problem in these cases has been the untenablp premise that
each couple acts quite independentlyof the other and is not affected by
the other's presence. However, as manyof these studies have shown, the
premise of additivitystill applies whenever the interactions have been
allowed for bycarrying out the electrochemical experiments in an appro-
priate fashion. The validityof adding or superimposing electrochemical
curves can therefore be considerablyextended by restating the principle
as follows:
Tùle 1
Summary of Recent Research to Test the Validity of the Wagner andA
Traud Additivity PrincipleA
a
Oxidant couple Reductant couple Electrode v exp /v mix Ref.
O /H O Cu/Cu 2+ Corroding Cu >1 10
2
2 2
2-
Cu(EDTA) /Cu HCHO/COO- Plating Cu >1 11
3- 4- - -
Fe(CN) 6 /Fe(CN) 6 I /I Anodized Pt 1 4
3
Reduced Pt <I 4
- -
3+
Fe /Fe 2+ I /I 3 Reduced Pt >1 3
Reduced Pt b 1 3
O 2 /H 2 O I - /I 2 Reduced Pt 0 3
Reduced Pt b (1) 3
- -
I /I Ti(III)/Ti(IV) Reduced Pt <1 16
3
Reduced Pt b 1 16
2+
Ce(IV)/Ce(III) Hg Hg 2+ Reduced Pt 1 17
2
Anodized Pt <1 17
2+
CO(NH 3 ) 5 Cl /Co 2+ DMPPD/è + Ag 1 19
Reduced Au or Pt <1 19
c
Reduced Au or Pt c 1 19
a v exp equals v corr , v plat or v cat, depending on the reaction studied.
b With the addition of a small concentration of iodide to the noniodine couple.=
c
With the addition of a small concentration of DMPPD to the cobalt couple,