Page 28 - MODERN ASPECTS OF ELECTROCHEMISTRY
P. 28
Voltaic Cells in Electrochemistry and Surface Chemistry of Liquids
α = µ i + z i Fχ s 15
s
s
i (2)
Relationships (1) and(2)togetherwith
M M M χ (3)
∆ S Ψ =∆ S ϕ – ∆ S –
M
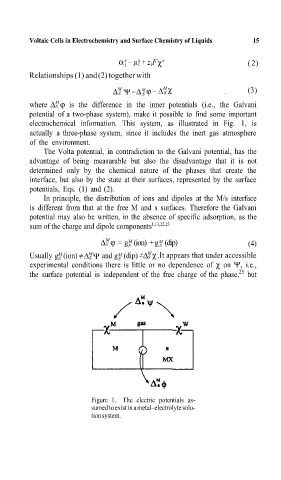
where ∆ S ϕ is the difference in the inner potentials (i.e., the Galvani
potential of a two-phase system), m¸e it possible to find some important
electrochemical information.= This system, as illustrated in Fig. 1, is
actually a three-phase system, since it includes the inert gas atmosphere
of the environment.=
The Volta potential, in contradiction to the Galvani potential, has the
advantage of being measurable but also the disadvantage that it is not
determined only by the chemical nature of the phases that create the
interface, but also by the state at their surfaces, represented by the surface
potentials, Eqs.= (1) and (2).
In principle, the distribution of ions and dipoles at the M/s interface
is different from that at the free M and s surfaces.= Therefore the Galvani
potential mayalso be written, in the absence of specific adsorption, as the
sum of the charge and dipole components 1,13,22,23
M
∆S ϕ =g (ion) +g (dip) (4)
M
M
S
S
Usuallyg (ion) = / ∆ S Ψ and g (dip) =/∆S χ.It appears that under accessible
M
W
M
M
S
S
experimental conditions there is little or no dependence of χ on Ψ, i.e.,
the surface potential is independent of the free charge of the phase, 25 but
Figure 1. The electric potentials as-
sumed to exist in a metal–electrolyte solu-
tion system.=