Page 31 - MODERN ASPECTS OF ELECTROCHEMISTRY
P. 31
18
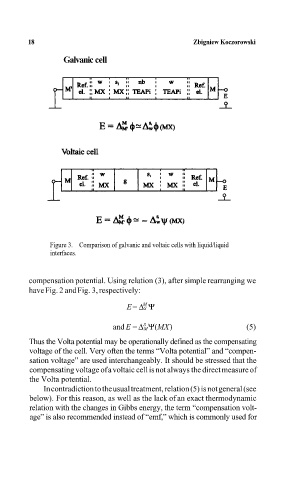
Galvanic cell Zbigniew KoczorowskiA
Figure 3. Comparison of galvanic and voltaic cells with liquid/liquid
interfaces.
compensation potential. Using relation (3), after simple rearranging we
have Fig. 2 and Fig. 3, respectively:
M Ψ
E= ∆S
and E = ∆ MΨ(MX) (5)
S
Thus the Volta potential may be operationally defined as the compensating
voltage of the cell. Very often the terms “Volta potential” and “compen-
sation voltage” are used interchangeably.=It should be stressed that the
compensating voltage of a voltaic cell is not always the direct measure of
the Volta potential.
In contradiction to the usual treatment, relation (5) is not general (see
below). For this reason, as well as the lack of an exact thermodynamic
relation with the changes in Gibbs energy, the term “compensation volt-
age” is also recommended instead of “emf,” which is commonly used for