Page 115 - MODERN ELECTROCHEMISTRY
P. 115
ION–SOLVENT INTERACTIONS 57
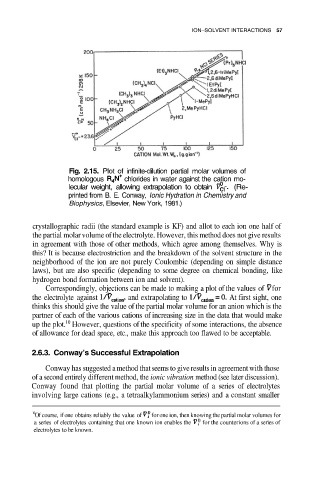
Fig. 2.15. Plot of infinite-dilution partial molar volumes of
homologous chlorides in water against the cation mo-
lecular weight, allowing extrapolation to obtain (Re-
printed from B. E. Conway, Ionic Hydration in Chemistry and
Biophysics, Elsevier, New York, 1981.)
crystallographic radii (the standard example is KF) and allot to each ion one half of
the partial molar volume of the electrolyte. However, this method does not give results
in agreement with those of other methods, which agree among themselves. Why is
this? It is because electrostriction and the breakdown of the solvent structure in the
neighborhood of the ion are not purely Coulombic (depending on simple distance
laws), but are also specific (depending to some degree on chemical bonding, like
hydrogen bond formation between ion and solvent).
Correspondingly, objections can be made to making a plot of the values of for
the electrolyte against and extrapolating to At first sight, one
thinks this should give the value of the partial molar volume for an anion which is the
partner of each of the various cations of increasing size in the data that would make
10
up the plot. However, questions of the specificity of some interactions, the absence
of allowance for dead space, etc., make this approach too flawed to be acceptable.
2.6.3. Conway’s Successful Extrapolation
Conway has suggested a method that seems to give results in agreement with those
of a second entirely different method, the ionic vibration method (see later discussion).
Conway found that plotting the partial molar volume of a series of electrolytes
involving large cations (e.g., a tetraalkylammonium series) and a constant smaller
10
Of course, if one obtains reliably the value of for one ion, then knowing the partial molar volumes for
a series of electrolytes containing that one known ion enables the for the counterions of a series of
electrolytes to be known.