Page 118 - MODERN ELECTROCHEMISTRY
P. 118
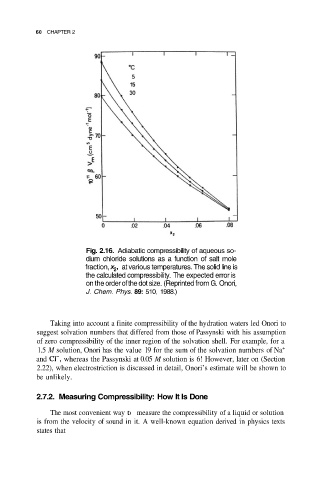
60 CHAPTER 2
Fig. 2.16. Adiabatic compressibility of aqueous so-
dium chloride solutions as a function of salt mole
fraction, at various temperatures. The solid line is
the calculated compressibility. The expected error is
on the order of the dot size. (Reprinted from G. Onori,
J. Chem. Phys. 89: 510, 1988.)
Taking into account a finite compressibility of the hydration waters led Onori to
suggest solvation numbers that differed from those of Passynski with his assumption
of zero compressibility of the inner region of the solvation shell. For example, for a
1.5 M solution, Onori has the value 19 for the sum of the solvation numbers of Na +
and whereas the Passynski at 0.05 M solution is 6! However, later on (Section
2.22), when electrostriction is discussed in detail, Onori’s estimate will be shown to
be unlikely.
2.7.2. Measuring Compressibility: How It Is Done
The most convenient way to measure the compressibility of a liquid or solution
is from the velocity of sound in it. A well-known equation derived in physics texts
states that