Page 123 - MODERN ELECTROCHEMISTRY
P. 123
ION–SOLVENT INTERACTIONS 65
Now the mass of the solvated ion is the sum of the mass of the bare ion,
and that of the primary solvation water, where is the solvation number of
the ion and is the molar weight of the solvent.
Thus:
If one takes this definition of and uses it in the above expression for one
obtains the rather lengthy expression:
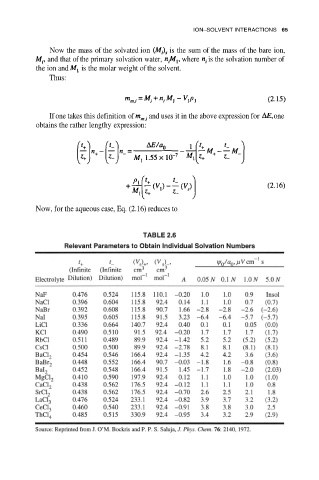
Now, for the aqueous case, Eq. (2.16) reduces to