Page 122 - MODERN ELECTROCHEMISTRY
P. 122
64 CHAPTER 2
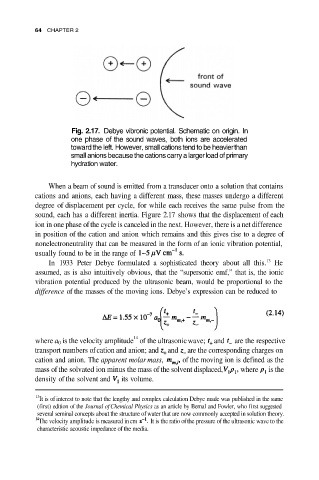
Fig. 2.17. Debye vibronic potential. Schematic on origin. In
one phase of the sound waves, both ions are accelerated
toward the left. However, small cations tend to be heavier than
small anions because the cations carry a larger load of primary
hydration water.
When a beam of sound is emitted from a transducer onto a solution that contains
cations and anions, each having a different mass, these masses undergo a different
degree of displacement per cycle, for while each receives the same pulse from the
sound, each has a different inertia. Figure 2.17 shows that the displacement of each
ion in one phase of the cycle is canceled in the next. However, there is a net difference
in position of the cation and anion which remains and this gives rise to a degree of
nonelectroneutrality that can be measured in the form of an ionic vibration potential,
usually found to be in the range of
13
In 1933 Peter Debye formulated a sophisticated theory about all this. He
assumed, as is also intuitively obvious, that the “supersonic emf,” that is, the ionic
vibration potential produced by the ultrasonic beam, would be proportional to the
difference of the masses of the moving ions. Debye’s expression can be reduced to
14
where a 0 is the velocity amplitude of the ultrasonic wave; and are the respective
transport numbers of cation and anion; and and are the corresponding charges on
cation and anion. The apparent molar mass, of the moving ion is defined as the
mass of the solvated ion minus the mass of the solvent displaced, where is the
density of the solvent and its volume.
13
It is of interest to note that the lengthy and complex calculation Debye made was published in the same
(first) edition of the Journal of Chemical Physics as an article by Bernal and Fowler, who first suggested
several seminal concepts about the structure of water that are now commonly accepted in solution theory.
14
The velocity amplitude is measured in cm It is the ratio of the pressure of the ultrasonic wave to the
characteristic acoustic impedance of the media.