Page 194 - MODERN ELECTROCHEMISTRY
P. 194
132 CHAPTER 2
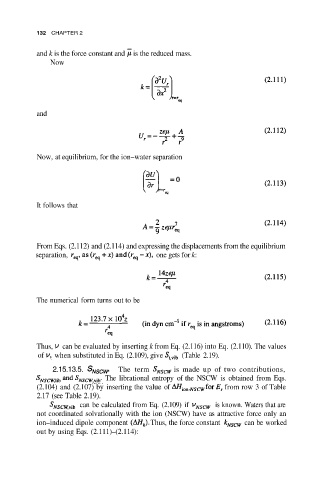
and k is the force constant and is the reduced mass.
Now
and
Now, at equilibrium, for the ion–water separation
It follows that
From Eqs. (2.112) and (2.114) and expressing the displacements from the equilibrium
separation, one gets for k:
The numerical form turns out to be
Thus, can be evaluated by inserting k from Eq. (2.116) into Eq. (2.110). The values
of when substituted in Eq. (2.109), give (Table 2.19).
2.15.13.5. The term is made up of two contributions,
The librational entropy of the NSCW is obtained from Eqs.
(2.104) and (2.107) by inserting the value of from row 3 of Table
2.17 (see Table 2.19).
can be calculated from Eq. (2.109) if is known. Waters that are
not coordinated solvationally with the ion (NSCW) have as attractive force only an
ion–induced dipole component Thus, the force constant can be worked
out by using Eqs. (2.111)–(2.114):