Page 232 - MODERN ELECTROCHEMISTRY
P. 232
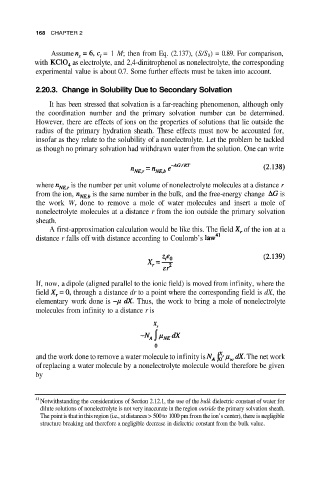
168 CHAPTER 2
Assume = 1 M; then from Eq. (2.137), (S/S 0) = 0.89. For comparison,
with as electrolyte, and 2,4-dinitrophenol as nonelectrolyte, the corresponding
experimental value is about 0.7. Some further effects must be taken into account.
2.20.3. Change in Solubility Due to Secondary Solvation
It has been stressed that solvation is a far-reaching phenomenon, although only
the coordination number and the primary solvation number can be determined.
However, there are effects of ions on the properties of solutions that lie outside the
radius of the primary hydration sheath. These effects must now be accounted for,
insofar as they relate to the solubility of a nonelectrolyte. Let the problem be tackled
as though no primary solvation had withdrawn water from the solution. One can write
where is the number per unit volume of nonelectrolyte molecules at a distance r
from the ion, is the same number in the bulk, and the free-energy change is
the work W r done to remove a mole of water molecules and insert a mole of
nonelectrolyte molecules at a distance r from the ion outside the primary solvation
sheath.
A first-approximation calculation would be like this. The field of the ion at a
distance r falls off with distance according to Coulomb’s
If, now, a dipole (aligned parallel to the ionic field) is moved from infinity, where the
field through a distance dr to a point where the corresponding field is dX, the
elementary work done is Thus, the work to bring a mole of nonelectrolyte
molecules from infinity to a distance r is
and the work done to remove a water molecule to infinity is The net work
of replacing a water molecule by a nonelectrolyte molecule would therefore be given
by
41
Notwithstanding the considerations of Section 2.12.1, the use of the bulk dielectric constant of water for
dilute solutions of nonelectrolyte is not very inaccurate in the region outside the primary solvation sheath.
The point is that in this region (i.e., at distances > 500 to 1000 pm from the ion’s center), there is negligible
structure breaking and therefore a negligible decrease in dielectric constant from the bulk value.