Page 236 - MODERN ELECTROCHEMISTRY
P. 236
172 CHAPTER 2
or:
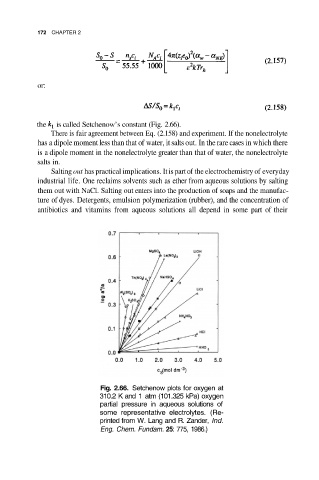
the is called Setchenow’s constant (Fig. 2.66).
There is fair agreement between Eq. (2.158) and experiment. If the nonelectrolyte
has a dipole moment less than that of water, it salts out. In the rare cases in which there
is a dipole moment in the nonelectrolyte greater than that of water, the nonelectrolyte
salts in.
Salting out has practical implications. It is partof the electrochemistry of everyday
industrial life. One reclaims solvents such as ether from aqueous solutions by salting
them out with NaCl. Salting out enters into the production of soaps and the manufac-
ture of dyes. Detergents, emulsion polymerization (rubber), and the concentration of
antibiotics and vitamins from aqueous solutions all depend in some part of their
Fig. 2.66. Setchenow plots for oxygen at
310.2 K and 1 atm (101.325 kPa) oxygen
partial pressure in aqueous solutions of
some representative electrolytes. (Re-
printed from W. Lang and R. Zander, Ind.
Eng. Chem. Fundam. 25: 775, 1986.)